 This week at ENA 2018, we’re presenting up to date research on our PDX models, including our newly developed prostate cancer PDX panel. We’re also presenting some systems biology results on tumor model and kidney cancer patient analysis.
This week at ENA 2018, we’re presenting up to date research on our PDX models, including our newly developed prostate cancer PDX panel. We’re also presenting some systems biology results on tumor model and kidney cancer patient analysis.
Prostate Cancer PDX Models for Preclinical Drug Development
ENA Poster 327: Evaluation of Anti-Androgen Therapy in a Panel of Prostate Patient-Derived Xenograft Models
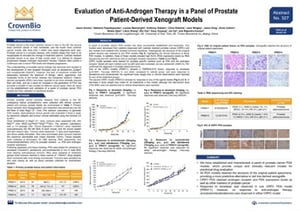 We’re delighted to present the latest treatment data on our newly developed prostate cancer patient-derived xenografts (PDX) at ENA this year. Prostate cancer PDX are difficult to develop due to issues with generation, propagation, and low take rates, so these models are a great new addition to our PDX collection.
We’re delighted to present the latest treatment data on our newly developed prostate cancer patient-derived xenografts (PDX) at ENA this year. Prostate cancer PDX are difficult to develop due to issues with generation, propagation, and low take rates, so these models are a great new addition to our PDX collection.
The poster details information on the original patients and primary prostate tissue for our first four models, as well as PDX establishment methods. We then focus on our two most highly-characterized castrate resistant prostate cancer (CRPC) models to look at their responses to standard of care docetaxel, as well as the newer targeted hormonal agents abiraterone and enzalutamide.
Prostate Cancer PDX Model Characterization
Before efficacy testing, the prostate cancer PDX models were characterized. H&E staining showed that our CRPC models retain the structure of the original patient specimens. RNA sequencing and IHC staining then looked at a variety of prostate cancer related markers. The models both have high KLK3 (PSA expression by RNAseq) and androgen receptor expression, with other molecular differences shown by IHC. All in all, these data make our models clinically relevant, capturing clinical disease features.
Prostate Cancer PDX Model Treatment Data
We’ve gone on to study model response to treatment. We saw varied response to docetaxel (significant response and resistance across the models), and no response for either model to abiraterone and enzalutamide. Different response to standard of care is likely due to the differences between models shown by the molecular characterization.
For the targeted agent resistance, we’re carrying on to study PSA and hormone levels to try and understand resistance mechanisms.
Overall, these new prostate cancer PDX models provide unique and clinically relevant resources for preclinical drug development.
Predicting Drug Response in Transplanted Tumor Models
ENA Poster 305: Evaluation of Categorical Response Methods in Predicting Drug Responses in Transplanted Tumor Models
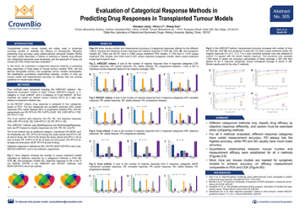 Tumor models, including PDX, are commonplace in preclinical oncology studies to assess agent efficacy. To analyze oncology study data, a variety of methods have been developed to classify drug efficacy into categorical responses. However, the different methods aren’t always consistent, classifying drug efficacy as objective response differently.
Tumor models, including PDX, are commonplace in preclinical oncology studies to assess agent efficacy. To analyze oncology study data, a variety of methods have been developed to classify drug efficacy into categorical responses. However, the different methods aren’t always consistent, classifying drug efficacy as objective response differently.
We decided to compare four different methods used to predict drug response in PDX as well as cell line-derived xenograft and syngeneic models. Our primary purpose was to develop best practices for comparing analysis datasets, though we also looked at mice number per model and how this effects accuracy to help provide guidance for mouse study design.
The four methods compared were:
- mRECIST criteria comprising complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD)
- RECIST criteria of CR, PR, SD, PD
- 3-category method of objective response (OR), SD, PD
- 5-category method of maintained complete response (MCR) CR, PR, SD, PD
All four methods are based on relative tumor volume, but with slightly different ways of classifying response into each category.
Measurement Accuracy in Different Response Categories
One important result was in the measurement accuracy across different response categories. For all the methods we tested, different response categories have varied measurement accuracy. For example, PD always had the highest accuracy, while PR and SD classifications usually had much lower accuracy.
Quantitative Mouse Number and Measurement Efficacy Relationships
For all four methods, we established quantitative relationships between mouse number and measurement efficacy. Measurement accuracy increases with the number of mice used across all the tumor model types studied. Interestingly, more mice per model are needed for syngeneics to achieve an efficacy measurement which is comparable to PDX and cell line derived xenografts.
Moving forward, this data can hopefully be used to guide better mouse tumor model study design and allow easier analysis of data when comparing results from multiple study types.
Genomic Characterization of Renal Cell Carcinoma Patient Populations
ENA Poster 312: Genomic Characterisation of Chinese Clear Cell Renal Cell Carcinoma Revealed Novel Prognostic Differences Across Populations
 Our final poster focused on the biological analysis of patient kidney cancer samples. Kidney cancer is one of the ten most common cancers for men and women in the US. There’s a lot of large-scale omics data available for kidney cancer patients, but this is mainly from Caucasian populations. As kidney cancer is also a concern in China, we looked at the genomics of a Chinese population, specifically clear cell renal cell carcinoma (ccRCC) patients.
Our final poster focused on the biological analysis of patient kidney cancer samples. Kidney cancer is one of the ten most common cancers for men and women in the US. There’s a lot of large-scale omics data available for kidney cancer patients, but this is mainly from Caucasian populations. As kidney cancer is also a concern in China, we looked at the genomics of a Chinese population, specifically clear cell renal cell carcinoma (ccRCC) patients.
Chinese ccRCC Genomic Analysis
Following whole transcriptome sequencing on 55 tumor tissues and 11 matched normal tissues, and comparison with TCGA Caucasian datasets, we found some interesting results.
Firstly, PBRM1 (which is often somatically mutated in ccRCC, a key driver mutation) is mutated with a much lower frequency in Chinese populations. Overall in the Chinese cohort, we found thirty-one gene fusion events, including five recurrent, a lot of which are associated with apoptosis, tumor suppression, and metastasis.
From our data, we could classify Chinese ccRCC patients into three cohorts with differing gene expression patterns:
- Class 1: significantly elevated VEGF pathway gene expression.
- Class 2: increased expression of extracellular matrix organization genes, strongly associated with high grade tumors and the worst patient survival.
- Class 3: significantly depleted VEGF pathway gene expression (a rare subtype in the TCGA cohort).
A final, important finding came from our analysis of the immune microenvironment of Chinese ccRCC patients. We found immune-active and tolerant tumors, with significantly increased macrophages and depleted CD4 positive T cells. This shows that some groups of patients may benefit from immunotherapy treatment, which is an important finding moving forward with ccRCC drug development.
More on ENA 2018
For more on our ENA 2018 presentations, check out these recent blog posts on our humanized and murine immuno-oncology posters.