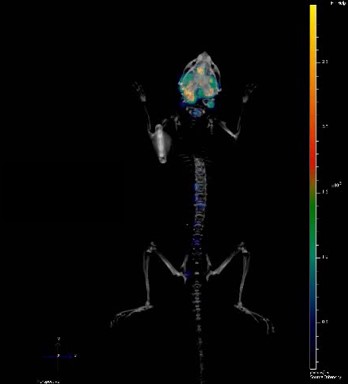
 Conventional xenografts are the first step in the in vivo oncology drug discovery process – for early stage research around PK/PD, ADME, toxicology studies etc. However, xenografts can also be modified to enhance and expand their research uses, including the development of bioluminescent models for optical imaging.
Conventional xenografts are the first step in the in vivo oncology drug discovery process – for early stage research around PK/PD, ADME, toxicology studies etc. However, xenografts can also be modified to enhance and expand their research uses, including the development of bioluminescent models for optical imaging.
Overall Benefits of Optical Imaging for Preclinical Research
Optical imaging including using bioluminescent models is particularly suited to preclinical oncology research, providing sensitive, cost-effective and high throughput in vivo tumor detection, monitoring, and follow-up. This allows more clinically relevant models through imaging, with a variety of tumor associated-properties being visualized in real time, non-invasively in living animal models. You can continuously monitor tumor burden and/or molecular events, with this continuous feedback letting you optimize your treatment regimens mid study.
Creation of Bioluminescent Models
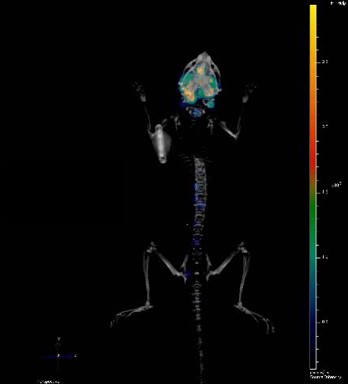
Before you can start imaging, you need to have a bioluminescent model. Creation can be fairly straightforward and fast. Cancer cell lines are homogenous cell populations which easily grow in 2D on plastic, and which are readily amenable to lentiviral transduction to express proteins such as firefly luciferase (the inventory of luciferases for in vivo BLI applications can be reviewed here).
Transduction is a great technique to use rather than transfection, as the consistency of transduction makes the selection of clones unnecessary. This method can produce a selected, transduced population in as little as 2 weeks. Clones can then be STR profiled to ensure a DNA profile match with the parental line which can take a further 3-4 weeks. The cell lines are then ready for any in vitro SoC testing and in vivo modeling studies.
Bioluminescent xenografts can be used subcutaneously, but the main utilities and usefulness come from orthotopic and metastatic models.
Orthotopic Models Mimic Primary Lesions
Orthotopic models mimic more patient-relevant primary lesions – tumor models are established with more relevant vasculature such as stroma, and interactions with appropriate microenvironments. Orthotopic models in general also provide more of a disease and human patient relevant response to standard of care treatments.
Bioluminescent orthotopic models allow progressive in-life tumor growth and treatment monitoring e.g. real time details on a compounds efficacy on a more clinically relevant tumor. Common endpoints in these studies include monitoring tumor burden (in vivo and ex vivo), tumor growth, and survival analyses.
Spontaneously Metastatic Models Mimic Cancer Progression
Bioluminescent models are extremely useful for metastatic disease research, allowing you to see where the tumor has metastasized, and if treatment has any effect. There is also establishment of clinically relevant vasculature and hypoxia in the primary orthotopic lesion.
Models can provide locoregional extension, lymphatic, and hematogenous metastasis, with metastatic site depending on the model used. Common metastatic sites from xenografts used in these studies tend to be similar to human disease – local lymph nodes, lung, liver, and bone.
Following single agent or combination treatment, study readouts can include primary tumor growth inhibition, tumor burden, and terminal tumor weight. Ex vivo imaging analyses can be used for both primary tumor and metastatic lesions, with results supported and confirmed by macroscopic and histological evaluation.
Experimental Metastasis Models Mimic Late Stage Disease
Experimental metastasis models allow modeling of the adaptation of metastatic end-stage disease without the restriction of primary lesions. These xenografts provide modeling of the hematogenous escape of tumor cells to distant seeding sites, and real time monitoring of resulting metastases.
Examples include bone metastasis following intracardiac injection of breast cancer models, which if treated correctly can also progress to brain cancer, mimicking human disease. This allows evaluation of agents specifically for metastatic disease, and in the case of brain cancer allows modeling of agents to cross the blood brain barrier. Endpoint readouts for these model types are similar to those for the models discussed above.
Don’t Forget Immuno-Oncology
While the focus of this blog was the use of bioluminescent xenografts, optical imaging can also be useful for immunotherapy, with bioluminescent metastatic syngeneic models available. Uses of these models include studying clinically relevant metastatic invasion, monitoring metastatic lesions in secondary organs, and the specific evaluation of immunotherapeutic agents to target this metastasis.
For more detailed information on the topics discussed in the blog:
Imaging preclinical tumour models: improving translational power. De Jong et al, Nature Reviews Cancer 14, 481–493 (2014)
Bioluminescent imaging: a critical tool in pre-clinical oncology research. O’Neill et al, The Journal of Pathology 220(3) 317-327 (2010)