Recent posts have covered the different types of mouse clinical trial (MCT) and which is most suited to any given drug development program. These posts all consider immunodeficient mice, and the progression of oncology agents such as targeted drugs or chemotherapy. However, MCT can also be used to progress immunotherapies, but require the use of humanized mice.
For researchers interested in running MCT with humanized mice, the current industry gold standard are humanized mice from The Jackson Laboratory. It should be noted that, in addition to the massive amount of work around development and ongoing characterization, there are limitations to these types of mouse models.
There is donor variability and that is something that needs to be considered when using humanized mice - that you are not going to get the same donor every time.
How Do You Choose Models and Design an Mouse Clinical Trial using Humanized Mice?
Within a patient-derived xenograft (PDX) collection where you have already identified your lead indication, PDX models can be screened for your specific checkpoint target expression (e.g. PD-1) and all appropriate models can be selected and recruited to a mouse clinical trial.
This is performed in a similar way to choosing models for a standard study, e.g. through a database of characterization data, TMAs etc.
1+1 Mouse Clinical Trials are Useful for Immuno-Oncology Studies
A common MCT design is 1+1, which has already been discussed in our previous posts: one animal for vehicle and one animal for your agent of interest.
Due to the variation between donors, when conducting humanized MCTs, it is best to have multiple PDX models in addition to multiple stem cell donors, in other words utilizing a checkerboard design. Endpoints for studies such as these would include:
- tumor growth inhibition
- tumor infiltrating lymphocyte assessment by flow cytometry
- as well as histology.
Use Checkerboard Design Studies to Overcome Variability in Response
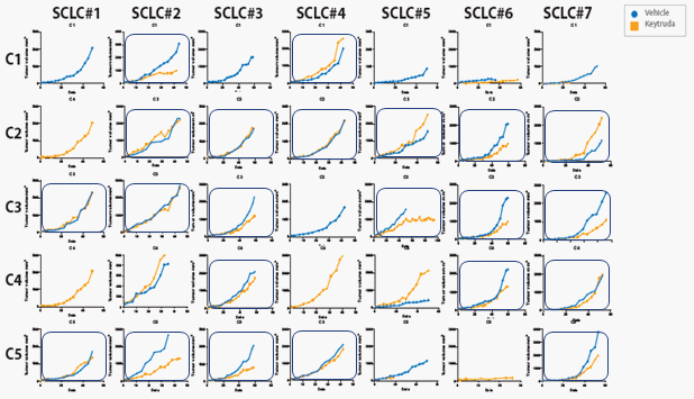
To help you visualize what a checkerboard designed study looks like your different PDX models would be positioned across (x-axis), with different donors shown down the side (y-axis).
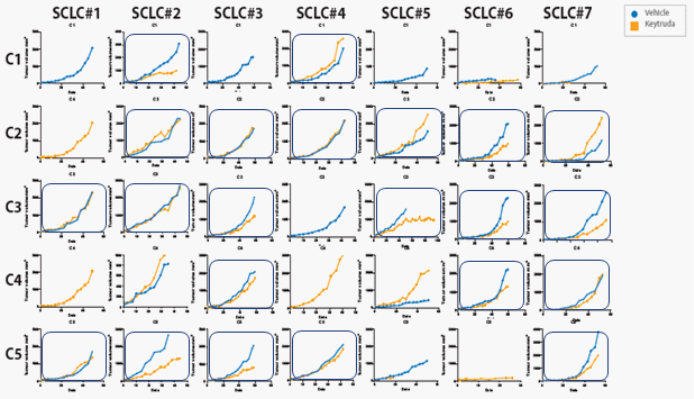 Anti-PD-1 response across a HSC donor population in SCLC PDX models.
Anti-PD-1 response across a HSC donor population in SCLC PDX models.

Anti-PD-1 response across a HSC donor population in SCLC PDX models
When utilizing this type of study design it becomes apparent that not a single model or donor will have a response across the board. So not a single model responds to all donors, nor a single donor responds with all PDX models. The only way to overcome this limitation is by doing these kind of checkerboard studies.
Risk of Redundant Data May Increase n Number
With these types of humanized models, you will always see dropouts and lose some animals, meaning some data may not be generated. Running a 1+1 study means there is always a risk – if you lose the vehicle or a treatment group that data set becomes redundant. However, you can always increase the n numbers per group to compensate for this effect.
Humanized MCTs are Useful for Advanced Drug Candidates
These types of checkerboard study designs are very useful to interrogate PDX models, especially when looking to investigate combination therapies as well as patient diversity. PDX models in addition to immuno-oncology models are now helping to answer the many questions being raised with the evolution of immunotherapeutics.
The humanized system (using reconstitution of the human system in mice) is something that is still in development and proving to be very relevant in providing positive results that can be correlated to the clinic.
Keeping in mind that the humanized mouse system is more for advanced assets, selecting these models should be done with careful consideration relative to the stage of your agent.
Further Reading
- 1+1 MCTs were fully validated by Novartis: Gao et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nature Medicine 2015;21(11): 1318-1325.