 Multiple sclerosis is a chronic disease affecting the central nervous system, which has multiple stages and currently no cure. This blog post reviews the background, mechanisms, and unmet needs of MS, as well as the complexity of common rodent models being used to develop new therapies for this autoimmune disease.
Multiple sclerosis is a chronic disease affecting the central nervous system, which has multiple stages and currently no cure. This blog post reviews the background, mechanisms, and unmet needs of MS, as well as the complexity of common rodent models being used to develop new therapies for this autoimmune disease.
What is Multiple Sclerosis?
Multiple sclerosis (MS) is an inflammatory autoimmune disease which affects the central nervous system (CNS), particularly the spinal cord. Both immune and nervous system cells are involved in the pathophysiology of the disease, resulting in myelin destruction and nerve damage. This leads to a wide range of motor and non-motor symptoms that vary among patients, including fatigue, spasticity, walking difficulties, problems with vision, speech, bladder and bowel dysfunctions, pain, cognitive and emotional changes, and depression.
Multifactorial Origins of MS
While the cause of MS is not known, it is considered as a heterogeneous disease with multifactorial origins. It is widely accepted that MS is the result of interactions of multiple genetic, infectious, and environmental factors, as well as compromised immune-mediated responses. This is particularly well-illustrated by studies on monozygotic twins, which can develop different disease severity or disease types.
Numerous studies illustrated several risk factors influencing MS susceptibility and disease course, including:
- Ultraviolet radiation
- Vitamin D
- Cigarette smoking
- Obesity
Infectious agents, especially Epstein-Barr virus infection, were also identified as risk factors.
Interestingly, recent studies also showed that the microbiome can influence disease outcome in rodent models of MS. An important observation, though, is that the microbiome has both disease-inducing and disease-protecting influences on CNS autoimmunity.
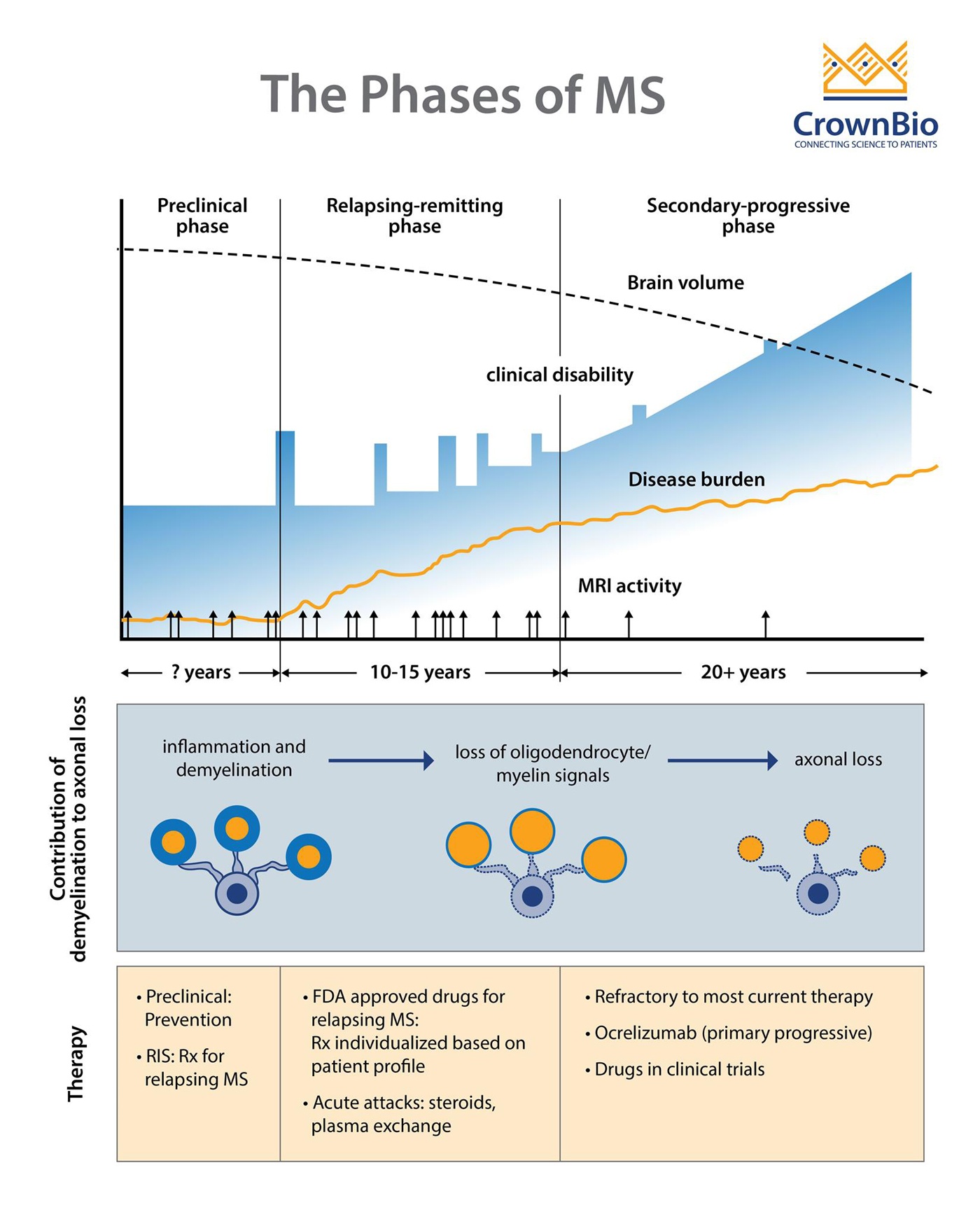
The Clinical Stages of MS
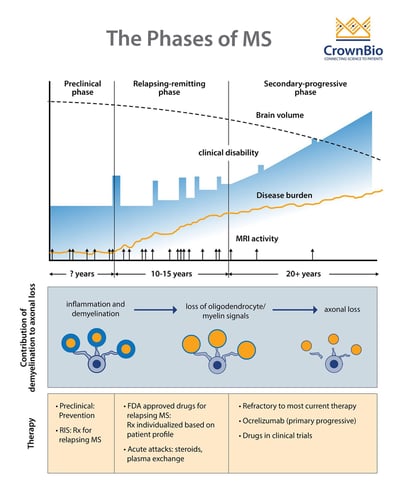
Adapted from Multiple Sclerosis, Disease Management website, Cleveland Clinic, Chandran et al. Phiilos Trans R Soc Lond B Biol Sci 2008;363(1489): 171-83, and Baecher-Allan et al. Neuron 2018;97(4):742-68.
Adapted from Multiple Sclerosis, Disease Management website, Cleveland Clinic, Chandran et al. Phiilos Trans R Soc Lond B Biol Sci 2008;363(1489): 171-83, and Baecher-Allan et al. Neuron 2018;97(4):742-68.
MS comprises three clinical stages:
- A preclinical stage detectable only by Magnetic Resonance Imaging (MRI)
- A relapsing-remitting (RRMS) stage characterized by episodes of neurologic dysfunction (optic neuritis, sensory and motor disturbances), followed by partial or complete resolution that can last months or years
- A progressive stage, which usually evolves from the relapsing stage and leads to progressively worsening of motor functions.
In some patients, MS can be progressive from onset and is called primary progressive disease (PPMS). It is currently unknown if PPMS represents a unique form of MS, or if it is a secondary progressive MS (SPMS) in which the relapse episodes were not apparent clinically. About 60-70% of individuals with PPMS develop SPMS.
Disease Mechanism of MS
The etiology of MS is unknown, but current hypotheses suggest an aberrant immune response against CNS antigens as the primary event. Following loss of peripheral tolerance, autoreactive T cells (CD8+, Th1, and Th17 cells), B cells, dendritic cells, and macrophages infiltrate the brain by crossing the blood brain barrier and, to a lesser extent, the blood cerebrospinal fluid barrier.
Recent evidence suggests that Th17 cells play an important role in blood-brain barrier breakdown by inducing reactive oxygen species within endothelial cells. Blood brain barrier breakdown further increases immune cell CNS infiltration and perivascular blood cerebrospinal fluid barrier immune cell accumulation.
Features of Early Stage MS
During the early disease stage, immune cells and activated, CNS-resident microglia and astrocytes promote progressive demyelination and degeneration of oligodendrocytes and axons. Various mechanisms are promoted to counter neural tissue injury.
Active lesions are common in RRMS (but become rare during progressive MS) and involve both the white and gray matter. In fact, cortical and deep grey matter atrophy are the best predictors of neurological disability in MS and have been associated with disease progression.
Features of Late Stage MS
During the late stage of the disease the capacity for tissue repair is lost. Immune cell infiltration declines, but CNS-mediated inflammation persists. Subpial lesions are relatively abundant in the progressive stage of MS and microglial activation is more important than peripheral immune cell infiltration.
Both macrophage and microglia accumulate at sites of active demyelination and neurodegeneration in MS. This negative environment, along with other factors, inhibits oligodendrocytes proliferation and differentiation and further promotes neurodegenerative processes.
Relapsing and Progressive MS Mechanisms
Relapsing MS is believed to be driven mostly by immune cells infiltrating the CNS. Therefore, therapies aimed at increasing the function of regulatory cells, or decreasing the number or function of effector cells and thus preventing trafficking of cells to the CNS, showed encouraging efficacy in relapsing MS.
Progressive MS includes both immune-dependent and immune-independent mechanisms. The immune-dependent mechanisms are driven by an innate immune response in the brain mediated by microglia, macrophages, and B cells, as well as lymphoid-like follicles in the meninges. The immune-independent mechanisms include mitochondria injury, oxidative stress, and ion imbalance.
Current therapies are not effective at treating progressive MS and new strategies addressing these mechanisms are necessary. Therapies aiming at restoring cell damage and demyelination are a critical need.
Mouse Models of MS
Given the complexity and heterogeneity of MS, the task of reproducing this disease in rodent models with some translational validity is not an easy one. Researchers have several options, depending on the clinical aspect or severity needed for their work.
Experimental Autoimmune Encephalomyelitis Models
The experimental autoimmune encephalomyelitis (EAE) model is the oldest and most commonly-used experimental model for human MS.
EAE recapitulates many aspects of the pathological features observed in MS, including:
- Inflammation
- Demyelination
- Axonal loss
- Gliosis
This model also offers the possibility of studying counter-regulatory mechanisms of resolution of inflammation and remyelination. However, each aspect of MS needs to be specifically developed in EAE models, making it sometime difficult to interpret and compare data. All this increases the difficulty in comparing data across studies and laboratories.
In addition, reagents used in those studies come from different sources and might induce different disease severity. However, many approved drugs, or agents in preclinical or clinical development, have been tested and validated on the basis of efficacy in EAE studies.
In mice, EAE is induced by an active immunization step after an experimental blood barrier insult using various concentrations of pertussis toxins. Sensitization to myelin antigens occurs through the use of adjuvant containing bacterial constituents capable of activating the innate immune system. Since the activating antigen in human is unknown, EAE is induced using a variety of CNS tissue antigens such as myelin basic protein (MBP), proteolipid protein (PLP139-151), or myelin oligodendrocyte glycoprotein peptide 35-55 (MOG35-55) in complete Freund’s adjuvant.
Disease incidence is high in susceptible animal stains and onset occurs after 9-12 days, followed by various pathological and clinical outcomes. The MOG35-55 model induces a monophasic, sustained form of EAE whereas the PLP139-151 and MBP models are characterized by the appearance of multiple phases of EAE reminiscent of RRMS.
Adoptive-Transfer EAE Models
Another model includes the adoptive-transfer EAE. In this case, myelin-specific CD4 T cells generated in donor animals by active immunization are injected in recipient immunocompromised animals. Interestingly, many findings observed in EAE studies are corroborated by results in MS.
Cuprizone Mouse Model of MS
Finally, the cuprizone mouse model bypasses the autoimmune component of EAE models and offers the alternative of studying demyelination and gliosis, or rapid proliferation of glial subtypes. Cuprizone, a copper chelator, can be administered orally and therefore is the most frequently used toxicant-induced MS model.
Unmet Needs in MS
Advances in various research disciplines (especially into neuro-immune mechanisms that contribute to MS) led to the development of FDA-approved drugs that target effector T cells, regulatory cells, B cells, and cell trafficking into the CNS. Most drugs are effective in RRMS; however, none are efficacious for progressive diseases.
One obvious reason for this discrepancy might be differences in biological mechanisms of the disease that are currently not fully understood. For instance, ocrelizumab, a B cell-depleting anti-CD20 monoclonal antibody was approved in March 2017 by the FDA for PPMS and reveals an interesting role for B cells in MS. A published by Baker et al, many drugs approved by the FDA to treat MS physically or functionally deplete memory B cells. In total, more than 100 drugs are currently approved by the FDA for MS mostly targeting immune cells.
Priorities in MS Drug Development
Given the complexity and heterogeneity of MS, clinical trials could be greatly improved by developing biomarkers for patient stratification, disease progression, and efficacy.
Advances in personalized medicine and imaging techniques, and a better understanding of the microbiome, will also contribute to this goal as well as improving disease physiopathology.