 Explore the power of HUB tumor organoids in predicting clinical response.
Explore the power of HUB tumor organoids in predicting clinical response.
The Impact of Better Prediction in Oncology Drug Discovery
Why does one cancer patient respond so well to a certain chemotherapy, and another patient - with a seemingly similar tumor - not? Why does immunotherapy work well in some cases, but fail miserably in others?
Cancer heterogeneity is one answer to this question, and a challenge that needs extensive modelling to be beaten. Being able to better predict, or even understand, a patient’s response to treatment would mean a great deal for both the pharmaceutical and the medical world.
First of all, using better (biomarker-) defined test-groups in clinical trials, drug discovery success rates would go up and more novel drugs would likely make it from the drawing board into the clinic. Moreover, down-the-line, allowing for better treatment decisions, patients would have a higher chance of survival and medical costs would go down.
Using More Predictive Preclinical Models
Preclinical models reflecting the vast patient variability and tumor heterogeneity play a big role in solving this problem. Extensive living biobanks of patient-derived tumor samples allow for screening drug responses of many different tumors. Learning more about the similarities and differences between tumors, both in response and in genetic and expression profiles, allows for predicting sensitivities and ultimately for identification of biomarkers.
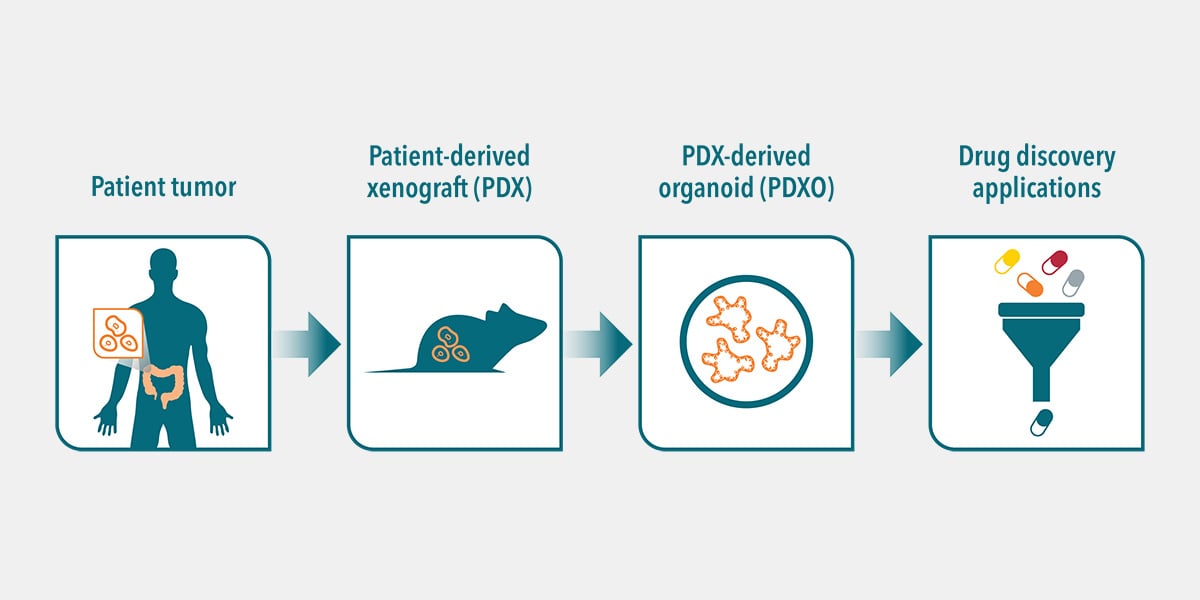
Next to well-established PDX models, patient-derived HUB organoids have recently been added to the portfolio of preclinical models that are highly suitable for predicting treatment responses in a high-throughput fashion.
HUB Organoid Characteristics
Characteristics that make HUB organoids stand-out and suitable for this purpose include:
- Genetically stable models, representing the original tumor, and available for (almost) any epithelial cancer type
- Expanding, well-characterized biobanks reflecting greater interpatient variability
- Relatively fast to use, amenable to high-throughput screening
- Amenable to co-cultures, allowing for prediction of immunotherapy responses
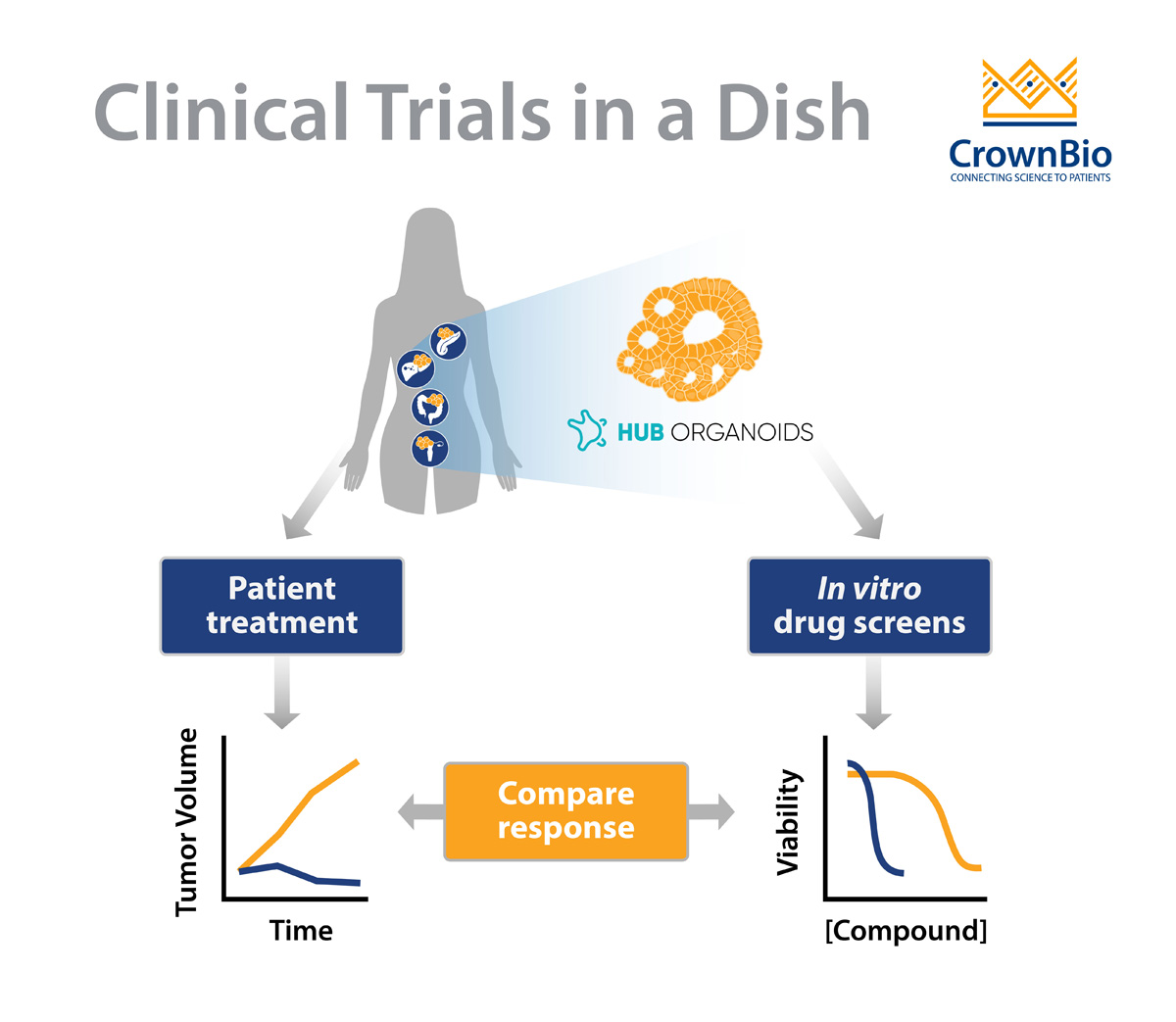
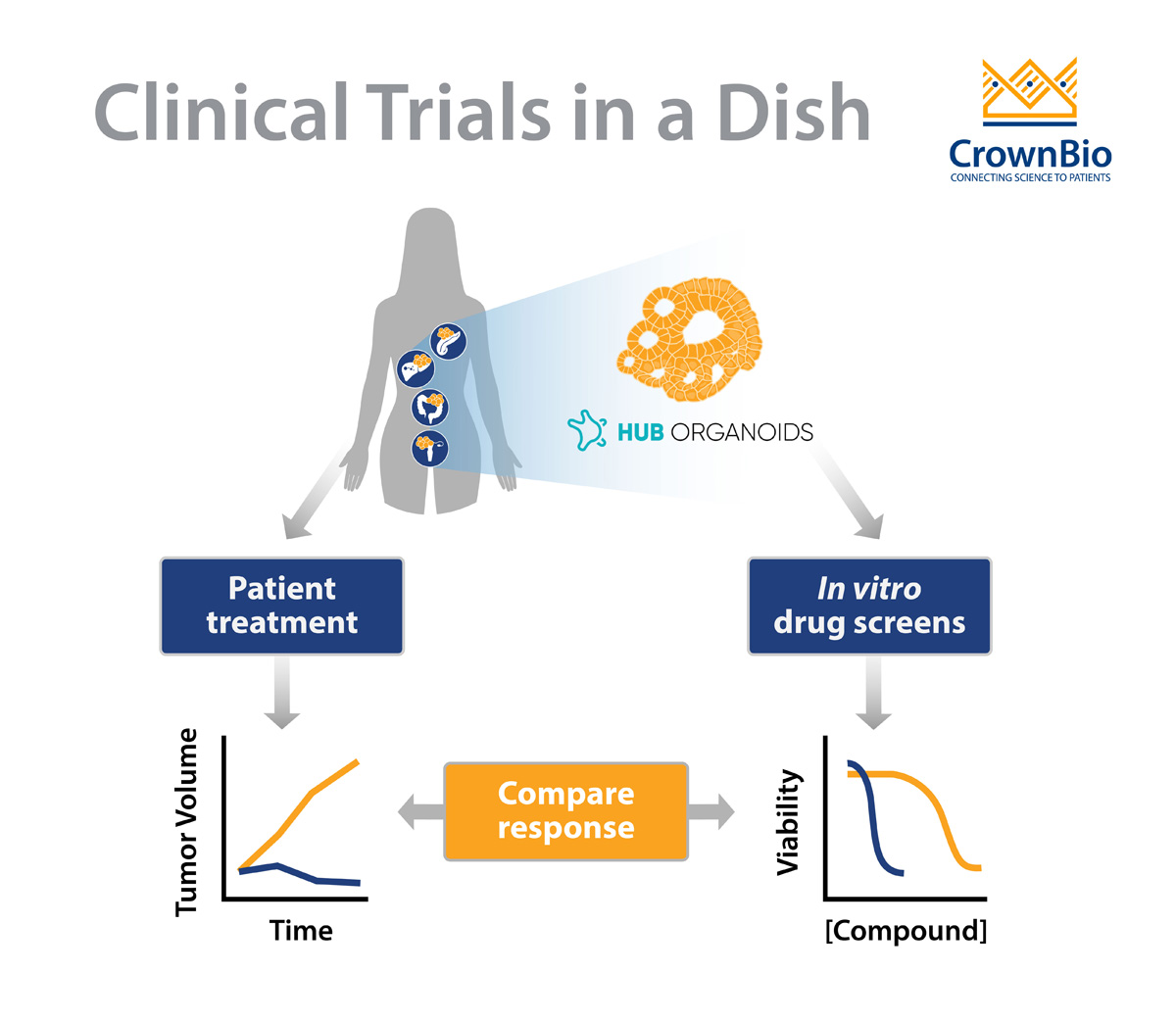
Besides these characteristics, it’s most important to highlight that organoids do indeed predict the patient’s clinical response.
Predictive Power of HUB Organoids: Diagnostic Tool
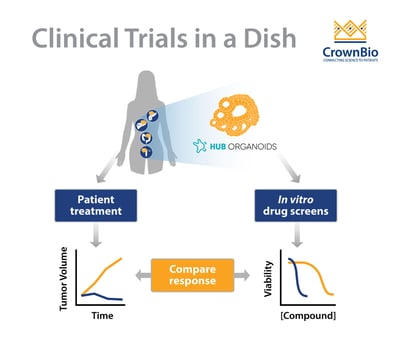
Several studies indicate a strong predictive potential in HUB organoids, and more (prospective) studies are expected to come out in the near future. The ultimate goal for many of these studies is to determine if organoids can be used as a diagnostic tool, to allow for selecting personalized medicine treatments. The fact that tumor organoids are actually viewed as potential diagnostic tools is indicative of the high predictive potential that is generally attributed to them.
What Does Science Say About Predicting Clinical Response?
Several recent studies have shown the predictive value of various types of HUB organoids to chemo- and/or radiotherapy.
For example, using a biobank generated from metastatic gastrointestinal tumors, researchers were able to predict clinical drug responses of 21 tumours to six different chemotherapies with great accuracy. Similarly, a study using locally advanced rectal organoids predicted treatment sensitivity of 68 patients to chemoradiation (combination of irradiation, 5-FU and irinotecan) with an accuracy of over 84%, findings which were backed up by another smaller study.
A study using colorectal (CRC) organoids confirmed they can predict sensitivity to irinotecan-based, but not to 5-FU/oxaliplatin combination therapy, indicating predictivity is not per se the same for all drugs. Here, other factors such as stroma or immune components might come into play; co-cultures of which are currently under development.
Besides these bigger studies, many similar findings have been made in smaller patient cohorts or at an anecdotal level. This way, tumor organoids have predicted clinical responses for:
- Gastric cancer
- Esophageal cancer
- Ovarian cancer
- Breast cancer
- Pancreatic cancer
- Head and neck cancer (radiotherapy)
Other Organoid Opportunities
Besides determining biomarkers for patient-group selection, the predictive potential of organoids also allows for the identification of novel drug sensitivities of particular cancer types. For example, several novel drug sensitivities have been identified for head and neck cancer and gastric cancer.
Clinical follow-up of these type of findings have not been published to date, but will be interesting to follow as they will allow the rapid expansion of clinical treatment toolkits. Vice versa, and maybe more relevant for drug-discovery processes, patient volumes could potentially be increased by testing drug sensitivities of a wide variety of indications, something that is very possible using organoid drug screens.
Conclusion
Many big and smaller studies have exemplified the strength of organoid drug screens for predicting clinical treatment responses. Outcomes of more preclinical studies (for each drug and each tumor type) will tell if organoids can live up to their diagnostic expectations.
In the meantime, the combination of extensive and well-characterised organoid biobanks and strong bioinformatic analysis/biomarker identification tools will prove to be a powerful tool for improving preclinical research outcomes.