How Do HUB Organoids Differ from Other Organoid Cultures?
 Explore the similarities and differences between the Hubrecht Organoid Technology (HUB) approach and other commonly used methods for establishing organoid cultures.
Explore the similarities and differences between the Hubrecht Organoid Technology (HUB) approach and other commonly used methods for establishing organoid cultures.
What is an Organoid?
The term ‘organoid’ was loosely used in the past to refer to 3D aggregates forming when cells were cultured in vitro in the absence of an attachment substrate. The current definition comes from the pioneering work of the Clevers lab, leading to the discovery of adult stem cells in the intestine.
Today, organoids specifically refer to structures with the following characteristics:
- A 3D structure derived from stem cells that establish and retain the identity of the organ being modeled (both stem cells and their progeny).
- Similar features to the organ which the organoid was derived from:
- Presence of multiple cell types.
- Some aspect of the organ’s specialized function.
- Self-organization which parallels the parental organ’s same intrinsic organizing principles.
Advancements in Organoid Technology
Great advances in organoid development have taken place in the last decade. With current organoid technology, long-term stem cell-based organotypic cultures are established without using feeder cells. Previously, feeder cells provided a cellular source of unknown (at the time) niche factors, supporting the ex vivo proliferation and expansion of adult epidermal stem cells and embryonic stem cells.
Using feeder cells, however, brought genetic stability issues. Stem cells grown in the presence of feeder cells become genetically unstable, often causing the loss of their full differentiation potential in vitro. The cultured (putative) stem cells need to be transplanted into mice to check whether they truly harbor the defining self-renewal and differentiation characteristics of a stem cell.
In the late 2000s, the first generation of murine organoid cultures was established using Lgr5+ small intestinal stem cells. This was largely made possible due to advances in understanding the stem cell niche and factors regulating stem cell self-renewal and differentiation. Other important factors in the first-generation organoid development were the discovery of Lgr5 as an intestinal stem cell marker and the observation that intestinal stem cells proliferate and can be long-lived in vivo.
In more recent years, the identity of critical stem cell niche factors has been further refined. Therefore, organoids are now reliably grown without feeder cells by simply supplementing the culture medium with a defined cocktail of stem cell niche factors.
How Are Organoids Derived?
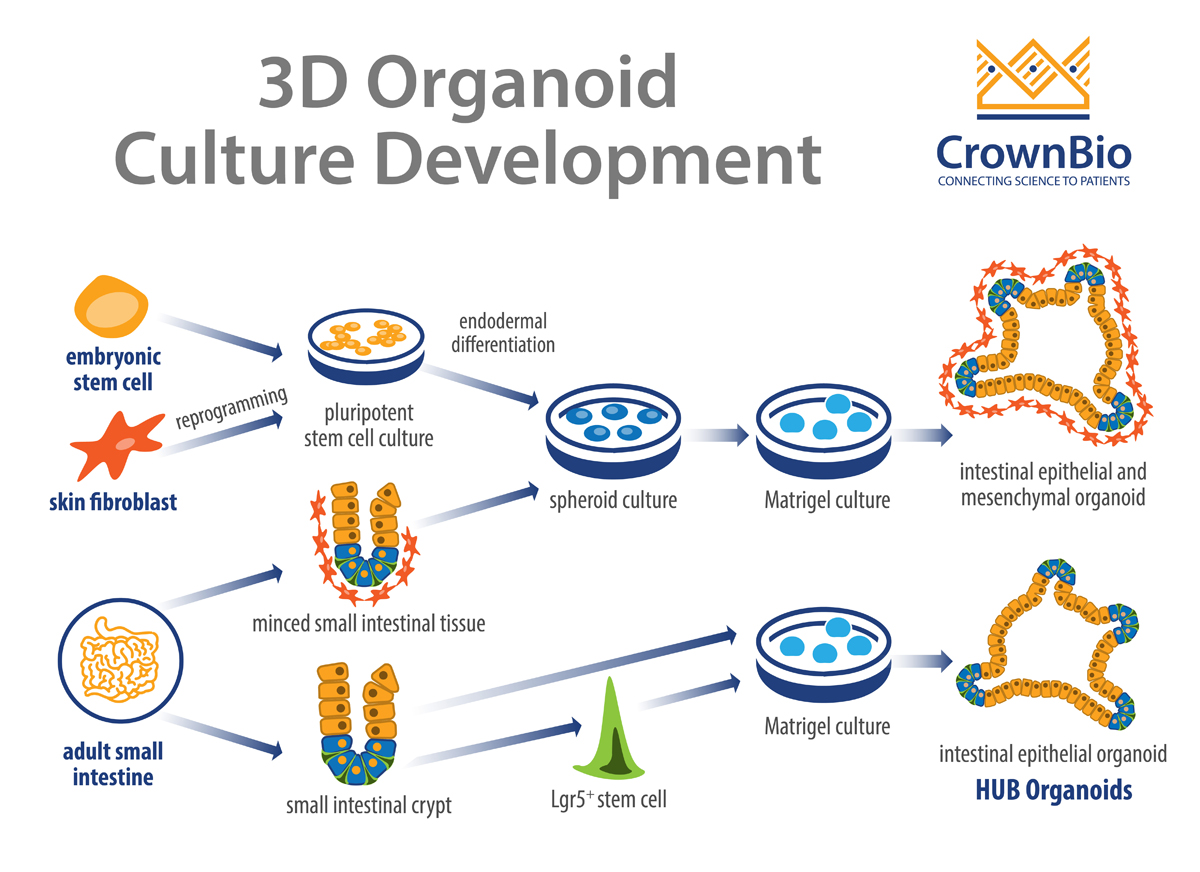
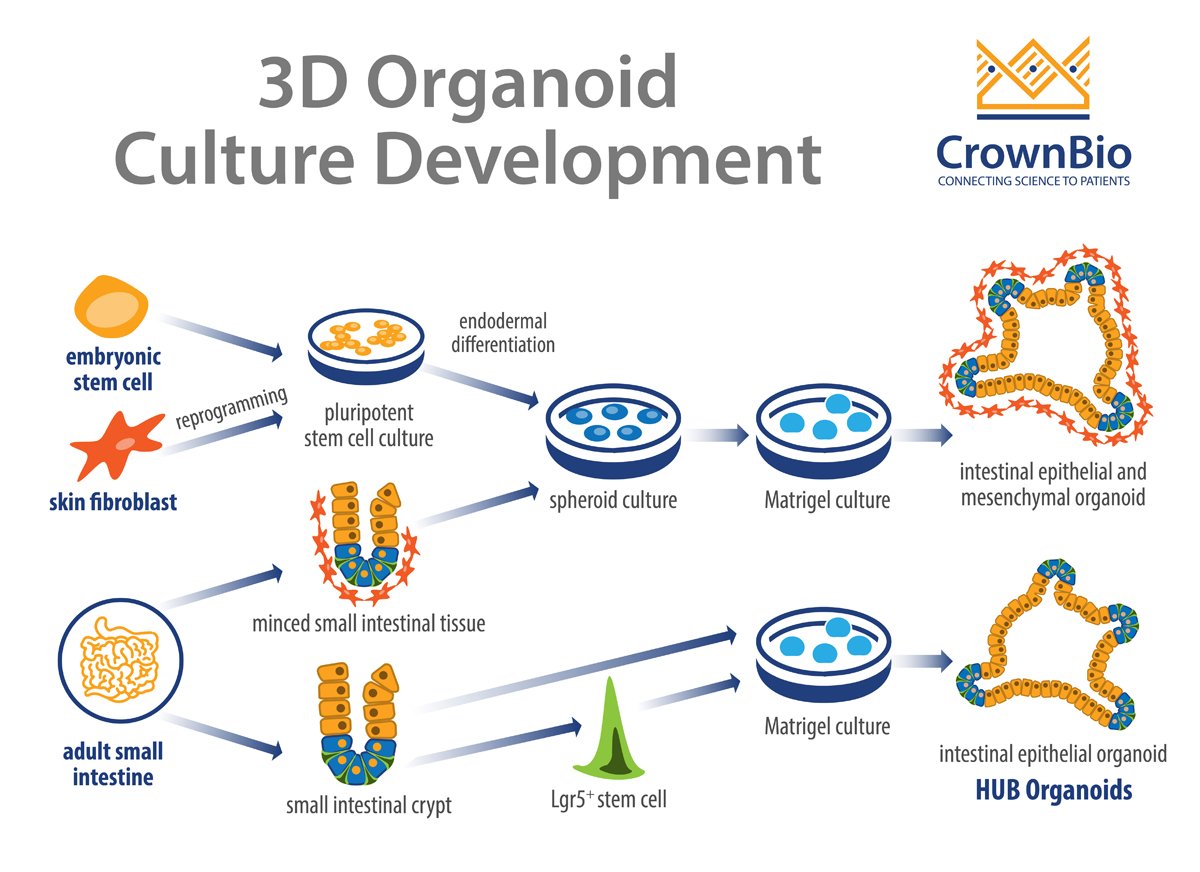
Organoids can be derived from either multipotent organ-specific adult stem cells (ASCs), pluripotent stem cells (PSCs) (i.e. embryonic stem cells, or ESCs), or induced PSCs (iPSCs). The resulting organoids can be composed exclusively of epithelial cells or both epithelial and mesenchymal cell types.
Adult Stem Cells
HUB was founded on the pioneering work of the Clevers lab and encompasses methods that are widely used and published. The core principle behind HUB Organoid technology is using purified single stem cells from adult tissue (identified by the presence of stem cell specific markers such as Lgr5) to derive fully developed organoids.
The Clevers group’s pioneering study constructed self-organizing and near-native intestinal epithelial structures without a mesenchymal cellular niche. By directly embedding single cells into Matrigel® in the presence of stem cell niche factors, epithelial organoid cultures are generated without a cellular mesenchymal niche.
The HUB protocols have been further refined to show that organoids can also originate from specific tissue compartments such as intestinal crypts. In this method, it’s believed that ASC are induced to reactivate their tissue regenerating potential. The trigger is the same signals that occur during a damage response following injury in vivo.
Other organoid techniques have been published which use minced epithelial tissue fragments supplemented by mesenchymal cells as a stem cell niche and grown on 3D collagen gels with an air-liquid interface.
Both of the above-mentioned techniques generate organoids with specific organ compartments such as intestinal crypts. However, HUB Organoids are considered to most closely resemble the adult organ of origin as they contain a full repertoire of fully differentiated cell types.
Pluripotent Stem Cells
When PSCs (and iPSCs) are used to derive organoids, the cellular cultures are first differentiated toward the germ layer from which the desired organoids were derived i.e. ecto-, endo-, and mesoderm. This is achieved by supplementing the culture with specific growth factors.
For example, when establishing intestinal organoids from PSCs, the PSC are treated with activin A to trigger the activation of transforming growth factor-β (TGF-β) signaling. This subsequently induces endodermal differentiation. The resultant self-aggregated clusters (i.e. spheroids) are then embedded into Matrigel and cultured in the presence of niche factors, such as epidermal growth factor (EGF), Noggin, and R-spondin-1. This results in organoid cultures that contain both epithelial and mesenchymal cells.
Organoids derived by this approach often recapitulate organogenesis, differentiating from premature tissues into near-native organotypic cultures that resemble their fetal counterparts. Intestinal organoid cultures have been successfully established using human PSCs, as well as human and mouse ESCs.
Benefits of HUB Organoids
From the different organoid types discussed above, HUB Organoids provide the broadest array of tissue identities, superior genomic integrity, and most well maintained long-term expansion and stability.
As described in a previous post, these beneficial features lead to improved modeling of healthy and diseased tissue biology and have positioned HUB Organoids as important tools in applications like large-scale drug screens.
For example, HUB Organoid stability has been a key factor in their use in drug screens. HUB tumor organoids closely recapitulate several properties of the original tumor, such as gene amplification, somatic copy number, and mutations. Each organoid represents the parental patient tumor and maintains the genetic and phenotypic characteristics. It was recently demonstrated that patient-derived organoids (PDOs) faithfully recapitulate patient response in the clinic and show great potential for use as a drug screening platform.
Additionally, compared to PSC or iPSC-derived organoids, HUB organoids are more quickly established as they don't require additional reprogramming and differentiation steps, or the passage of spheroid aggregates to Matrigel.
Overall, the close recapitulation of the organ of origin allows better understanding of the mechanisms of human development and disease. It also allows modeling of human biology and disease mechanisms that cannot be accurately modeled in animals because of evolutionary divergence.
Summary
Organoids are 3D structures derived from stem cells and composed of organ-specific cell types that have self-organized following endogenous developmental cues. However, not all organoids are equal. Recent advances in organoid technology have led to superior ex vivo models that better recapitulate the properties of the organ of origin.
HUB Organoid technology is based on protocols that have been refined and optimized to offer long-term cultures of ASC derived structures. HUB Organoids preserve genomic and phenotypic identity, and most faithfully recapitulate the multi-lineage differentiated identity of an organ in vivo. These models offer important benefits to study both human biology and disease mechanisms.
Cite this Article
Pal, R., (2019) How Do HUB Organoids Differ from Other Organoid Cultures? - Crown Bioscience. https://blog.crownbio.com/organoid-culture-comparison