The incidence of pancreatic cancer has been rising by about 1% per year over the past few decades, and it now accounts for ~3% of all cancers and 7% of cancer mortality in the U.S. Pancreatic cancer is now the third leading cause of cancer-related death, and it is exceptionally lethal in part because it is challenging to detect at an early stage. By the time signs and symptoms appear, the disease has often already reached an advanced stage and metastasized. The 5-year survival rate is dismal at 12%, and no clinically relevant diagnostic test can reliably detect early-stage pancreatic cancer in individuals without symptoms.
Scientists at JSR Life Sciences (JLS), the parent company of Crown Bioscience, have developed a novel and unique in vitro pancreatic cancer early detection technology (iv-PCED) that has great potential for detecting pancreatic cancer in its early stages.
The Value of Early Cancer Detection
This novel iv-PCED can play a significant role in the growing field of early cancer detection, for example, akin to other commercially available cancer diagnostic tests. Since survival greatly improves when cancer is detected early and therapies becoming increasingly expensive, there is strong rationale for payors to reimburse for such technologies and ensure proper use of therapeutics. Furthermore, early cancer detection can provide healthcare providers with valuable insights that can guide optimal care for their patients, who are generally becoming better informed, empowered, and tech savvy and want access to better diagnostic (Dx) testing options. The Dx landscape for early cancer detection is evolving rapidly with improved quality and sensitivity, and presents exciting growth opportunities. Companies active in the field of genetic cancer diagnostic technology can benefit on next generation sequencing (NGS) as a powerful technology to detect genomic alterations.
Increasing Role of NGS in Clinical Cancer Research and Care
For highly complex diseases such as cancer, NGS is positioned to play an increasingly important role in diagnosis, prognosis, and therapeutic testing (Figure 1). Furthermore, interest and opportunity are increasing for liquid biopsies which are blood-based tests that can detect cancer cells or DNA circulating in the blood and can reduce reliance on highly invasive surgical biopsies. Since early detection of cancer is intimately tied to better outcomes and survival, it is no surprise that several NGS-based multigene panels have been developed commercially.
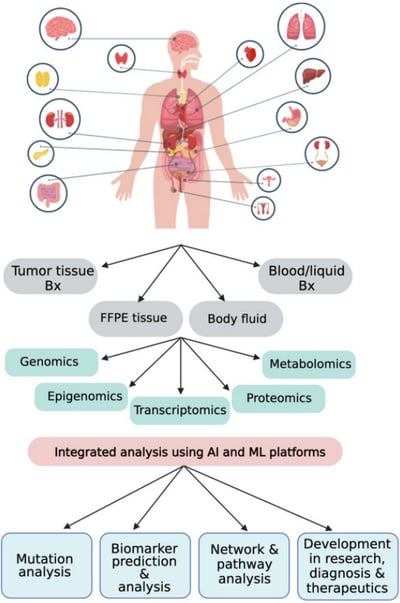
Figure 1: Role of NGS technology in cancer diagnosis, prognosis, and therapeutics using an integrative omics approach. From Satam, H. et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12(7), 997. Available at: https://doi.org/10.3390/biology12070997. Used under Creative Commons Attribution 4.0 International License: https://creativecommons.org/licenses/by/4.0/.
Novel in vitro Pancreatic Cancer Early Detection Test (iv-PCED)
This novel technology is based on a newly identified and validated panel of biomarkers that are relevant for the early diagnosis of pancreatic cancer. This novel approach, protected by intellectual property, uses a unique genetic cancer biomarker panel consisting of 23 specific fusion points shown to be relevant, in combination with an integrated and proprietary artificial intelligence (AI) algorithm to analyze the fusion points and provide valuable insights. This biomarker panel and companion AI holds great commercial potential as a Dx tool, such as for an in vitro PCED test (Figure 2).
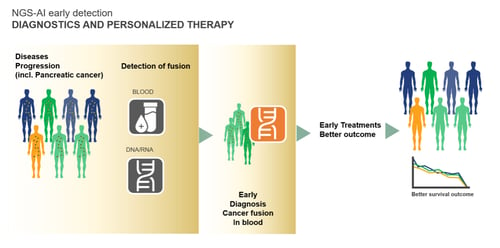
Figure 2: Potential Use of the iv-PCED.
Based on preliminary analyses, the JLS iv-PCED test is predicted to have high selectivity and specificity and may be used as a Direct-to-Consumer (DtC) Dx test in asymptomatic, high-risk individuals (such as those older than 50) and/or a medical Dx test for younger individuals with a family history of pancreatic cancer. The table below highlights the target product profile of the asset (Table 1).
| Primary | Secondary | |
|---|---|---|
| Product Type | In vitro Early Detection Genetic Diagnostic Test | In vitro Medical Genetic Diagnostic Test |
| Biomarker Type | Diagnostic biomarker Predictive biomarker |
Prognostic biomarker Monitoring biomarker |
| Indication | Pancreatic cancer (PCa) | |
| Unmet Need | High; PCa is 3rd leading cause of cancer-related death partly due to lack of early diagnosis testing | |
| Product Use | Direct-to-Consumer Genetic in vitro Pancreatic Cancer Early Diagnostic (IV-PCED) Test |
Medical Genetic Diagnostic Test for selection of best treatment regimens in PCa. |
| Target Population | Asymptomatic population at higher risk of cancer (>50 years old) |
Population with an associated family history of pancreatic cancer |
| Genetic Biomarker | A set of 25 biomarker fusion points occurring exclusively in Pancreatic cancer. Detection of one |
|
| Intellectual Property | Patent filing priority date Sept 8, 2021; WO2023039058 | |
| Stage of Development | Biomarker discovery | |
| Method of Detection | In tumor: WGS short reads; in blood: to be developed (WGS short reads or PCR-Amplicon) | |
| Competition | None for pancreatic cancer early detection test | |
Table 1: Target Product Profile of the iv-PCED.
Potential Out-licensing Opportunity
Crown Bioscience is currently seeking partners to out-license this technology platform and is open to negotiating a variety of possible out-licensing models. For instance, our collaborative licensing model offers to the licensee a creditable note against up-front and milestone payments on expenses incurred with JLS affiliated companies (such as Crown Bioscience and MBL) related to the development of the iv-PCED test.
To learn more about this unique out-licensing opportunity, please contact outlicensing@crownbio.com