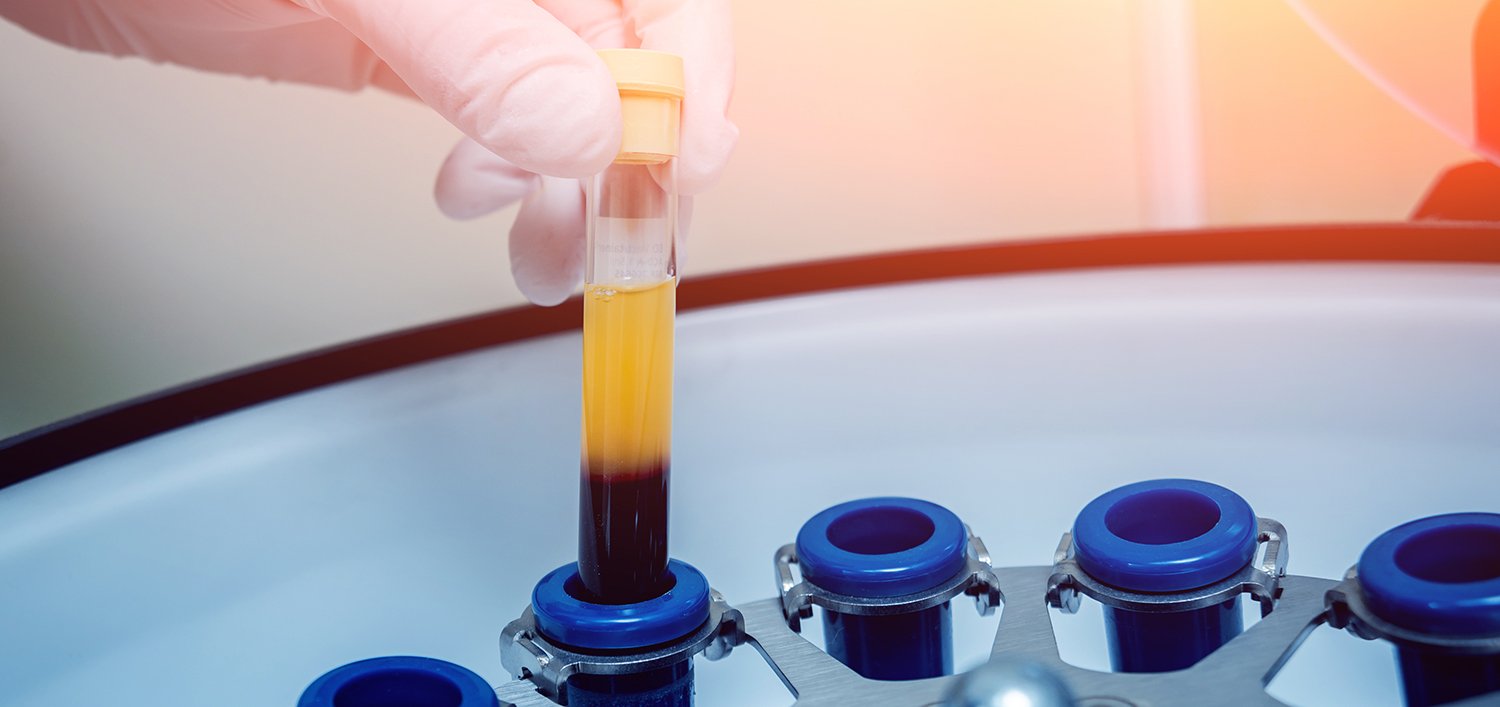
Cancer research has undergone remarkable advancements over the past decade, with an increasing focus on precision medicine and non-invasive diagnostic techniques. Among these innovations, longitudinal plasma analysis has emerged as a transformative approach in oncology, offering real-time insights into disease progression, treatment response, and biomarker discovery.
Unlike traditional tissue biopsies, which are invasive and provide only a single snapshot of tumor biology, longitudinal plasma analysis enables repeated sampling of circulating biomarkers from a patient’s blood over time. This technique allows researchers and clinicians to monitor circulating tumor DNA (ctDNA), proteins, and other molecular markers, facilitating early detection of relapse, therapeutic adjustments, and an improved understanding of cancer dynamics.
As oncology continues to shift toward personalized treatment strategies, longitudinal plasma is becoming an essential tool for guiding clinical decisions, refining therapeutic approaches, and enhancing drug development. This article explores the significance of longitudinal plasma in oncology research, its applications in biomarker discovery and personalized medicine, emerging technologies that support its growth, and the challenges that must be overcome to fully integrate it into clinical practice.
Understanding Longitudinal Plasma
What is Longitudinal Plasma?
Longitudinal plasma refers to the systematic collection and analysis of blood plasma samples from the same individual at multiple time points over a period of weeks, months, or even years. This approach provides a dynamic, real-time perspective on molecular changes within a patient’s body, unlike static, single-timepoint analyses, which only offer a limited snapshot of a disease at one specific moment.
Plasma, the liquid component of blood, contains various circulating biomarkers that provide critical insights into disease status, progression, and treatment response. In oncology, the study of longitudinal plasma helps researchers and clinicians track how tumors evolve over time, identify emerging resistance mechanisms, and tailor treatments accordingly.
Key biomarkers detected in plasma include:
- Circulating Tumor DNA (ctDNA): Short fragments of tumor-derived DNA released into the bloodstream as cancer cells undergo apoptosis or necrosis. Tracking ctDNA levels over time allows for early detection of tumor recurrence, monitoring of residual disease, and identification of resistance mutations.
- Circulating Tumor Cells (CTCs): Intact tumor cells that have shed into circulation from the primary tumor or metastatic sites. Studying CTCs over time can provide valuable information about tumor heterogeneity, metastatic potential, and treatment response.
- Extracellular Vesicles (EVs) and Exosomes: Small membrane-bound particles secreted by tumor cells that carry RNA, DNA, proteins, and lipids. These vesicles facilitate tumor communication and provide non-invasive access to tumor-specific molecular changes.
- Metabolites and Cytokines: Molecules involved in tumor metabolism and immune system interactions, which can help in tracking cancer progression, immune evasion, and inflammation-related responses.
By analyzing these biomarkers over multiple time points, longitudinal plasma studies help researchers uncover subtle molecular shifts that might signal tumor growth, relapse, or therapeutic resistance before they become clinically detectable through imaging or traditional biopsies.
Why Longitudinal Plasma is Superior to Single-Timepoint Analysis
Traditional approaches to cancer diagnosis and monitoring typically rely on:
- Tissue Biopsies: Invasive procedures that remove a sample of tumor tissue for analysis. While biopsies provide direct tumor-specific data, they have significant limitations:
- They are invasive and painful, limiting patient compliance.
- They provide only a single snapshot of the tumor at one point in time.
- They may not capture tumor heterogeneity, as a single biopsy might not reflect genetic variations across different tumor regions.
- One-Time Blood Tests: While these offer a less invasive alternative, a single blood test provides only a momentary glimpse into circulating tumor biomarkers, potentially missing important changes occurring between sample collections.
Longitudinal plasma analysis overcomes these limitations by offering continuous, real-time molecular insights into tumor evolution. Key advantages include:
- Real-Time Tracking of Tumor Dynamics
Longitudinal plasma enables frequent and non-invasive monitoring of tumor-derived biomarkers, allowing for a more accurate understanding of cancer progression, treatment response, and the emergence of resistance mutations. This is particularly useful for tracking minimal residual disease (MRD) and guiding adaptive treatment strategies.
- Early Detection of Resistance Mechanisms
Cancer cells often acquire genetic mutations that make them resistant to targeted therapies. Longitudinal plasma analysis can detect these mutations in ctDNA before clinical symptoms appear, allowing oncologists to adjust treatment plans proactively. For example:
- In lung cancer, EGFR T790M mutations can indicate resistance to first-generation EGFR inhibitors, guiding a switch to second-line therapies.
- In colorectal cancer, KRAS mutations emerging in plasma may signal resistance to anti-EGFR therapies, prompting a change in treatment approach.
- Minimized Need for Invasive Tissue Biopsies
Since longitudinal plasma can be collected through simple blood draws, it significantly reduces the reliance on invasive tissue biopsies, improving patient comfort and compliance. This is especially important in metastatic cancers, where obtaining tissue samples from multiple sites is often impractical.
- Better Assessment of Minimal Residual Disease (MRD)
Minimal residual disease refers to the small number of cancer cells that remain in a patient’s body after treatment and may lead to relapse. Longitudinal plasma enables early MRD detection by tracking ctDNA levels, offering a more sensitive alternative to imaging techniques that may miss microscopic disease.
- More Accurate Predictions of Treatment Response
By analyzing plasma biomarkers before, during, and after treatment, clinicians can determine:
- Whether a therapy is working as intended.
- Whether the cancer is adapting to the treatment and developing resistance.
- If an alternative therapy should be considered before clinical progression occurs.
For example, in breast cancer patients receiving chemotherapy, a drop in ctDNA levels post-treatment is associated with better outcomes, whereas persistently high ctDNA levels may indicate poor response and a need for treatment modification.
Impact of Longitudinal Plasma on Clinical Decision-Making
The ability to analyze longitudinal plasma samples has had profound implications for clinical oncology, particularly in:
- Personalized Medicine:
- Helps tailor treatments based on evolving tumor biology.
- Enables early intervention before drug resistance fully develops.
- Monitoring Cancer Remission & Recurrence:
- Detects relapse earlier than traditional imaging.
- Improves surveillance strategies for high-risk patients.
- Optimizing Clinical Trials & Drug Development:
- Enhances patient selection by identifying those most likely to respond.
- Reduces trial costs by minimizing the need for repeat biopsies.
Longitudinal plasma is transforming cancer research and treatment by offering a minimally invasive, real-time approach to tracking tumor evolution. Unlike single-timepoint analyses, longitudinal plasma provides continuous molecular insights, enabling early detection of resistance, improved treatment response monitoring, and better long-term disease management.
As technology advances—with improvements in liquid biopsy techniques, AI-driven biomarker discovery, and multi-omics approaches—longitudinal plasma is set to become an essential tool in precision oncology. Its widespread clinical adoption will ultimately enhance patient outcomes, refine therapeutic strategies, and revolutionize cancer care worldwide.
Role of Longitudinal Plasma in Oncology Research
Longitudinal plasma analysis has emerged as a powerful tool in oncology research, revolutionizing how clinicians and researchers track tumor progression, assess treatment efficacy, and guide therapeutic decisions. By leveraging circulating biomarkers, such as circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and extracellular vesicles (EVs), longitudinal plasma provides real-time, non-invasive insights into tumor biology. This continuous monitoring approach is proving instrumental in biomarker discovery, personalized medicine, early cancer detection, and clinical trials, ultimately enhancing patient outcomes.
Biomarker Discovery & Monitoring
One of the most significant contributions of longitudinal plasma to oncology is its ability to identify and monitor biomarkers that reflect tumor burden, progression, and response to therapy. Traditional biomarker assessment methods, such as tissue biopsies, often fail to capture the dynamic nature of cancer, whereas plasma-based biomarker monitoring provides continuous insights with minimal patient burden.
The Role of Circulating Tumor DNA (ctDNA) in Biomarker Discovery
ctDNA, which consists of fragmented tumor DNA released into the bloodstream, has become one of the most clinically valuable biomarkers for detecting mutations, tracking tumor evolution, and monitoring therapy response.
Key Applications of ctDNA in Different Cancers:
- Non-Small Cell Lung Cancer (NSCLC):
- Detecting EGFR mutations in ctDNA helps oncologists determine eligibility for tyrosine kinase inhibitors (TKIs) such as osimertinib or erlotinib.
- The emergence of EGFR T790M mutations can signal acquired resistance to first-line EGFR inhibitors, prompting a shift to second-line therapies.
- Breast Cancer:
- Monitoring PIK3CA mutations in ctDNA helps determine whether patients can benefit from PI3K inhibitors (e.g., alpelisib).
- Tracking ESR1 mutations in estrogen receptor-positive (ER+) breast cancer can guide decisions about hormonal therapy resistance and the need for alternative treatments.
- Colorectal Cancer:
- KRAS and BRAF mutations detected in plasma can indicate resistance to anti-EGFR monoclonal antibodies (e.g., cetuximab, panitumumab).
- The detection of MSI (microsatellite instability) in plasma can help determine eligibility for immune checkpoint inhibitors.
Personalized Medicine Applications
Personalized medicine aims to tailor treatment strategies to individual patients based on their unique tumor biology. Longitudinal plasma analysis plays a crucial role in this approach by allowing oncologists to continuously monitor molecular changes and adapt treatments in real time.
How Longitudinal Plasma Supports Personalized Oncology:
- Adjusting Therapies Based on Tumor Evolution
- Longitudinal plasma enables early detection of genetic alterations that may influence drug sensitivity or resistance, allowing oncologists to adjust therapy regimens accordingly.
- Example: A patient with BRAF-mutant melanoma who initially responds to BRAF inhibitors may develop resistance due to secondary MEK pathway mutations, detectable in ctDNA.
- Detecting Emerging Drug Resistance Before Clinical Symptoms Appear
- Resistance to targeted therapies often develops months before it becomes evident in imaging scans. Longitudinal plasma enables early detection of new mutations that confer drug resistance, allowing oncologists to switch therapies proactively.
- Example: In prostate cancer, the detection of AR-V7 splice variants in plasma suggests resistance to androgen receptor-targeted therapies, such as enzalutamide and abiraterone, prompting a shift to chemotherapy.
- Monitoring Immune Response in Immunotherapy
- Immunotherapy success is often unpredictable, and biomarker monitoring in plasma helps assess whether patients will benefit from immune checkpoint inhibitors.
- Example:
- PD-L1 expression levels in plasma can indicate likelihood of response to anti-PD-1/PD-L1 therapies (e.g., pembrolizumab, nivolumab).
- Tumor Mutational Burden (TMB) in plasma correlates with response to immune checkpoint inhibitors, guiding treatment selection.
These examples demonstrate how ctDNA-driven biomarker monitoring through longitudinal plasma allows for early intervention, improved treatment planning, and reduced reliance on invasive biopsies.
These applications demonstrate how longitudinal plasma enables precision medicine by allowing oncologists to track molecular changes, predict resistance, and optimize treatment strategies in real time.
Early Cancer Detection & Minimal Residual Disease (MRD) Monitoring
Cancer recurrence remains a major challenge in oncology, as traditional imaging methods often fail to detect minimal residual disease (MRD)—small numbers of remaining cancer cells that can lead to relapse. Longitudinal plasma analysis offers a highly sensitive method for detecting MRD and predicting relapse much earlier than conventional techniques.
How Longitudinal Plasma Improves Early Cancer Detection & MRD Monitoring:
- Leukemia and Lymphoma:
- MRD detection via ctDNA and next-generation sequencing (NGS) allows clinicians to determine whether a patient has achieved complete remission or requires further treatment.
- Example:
- Acute lymphoblastic leukemia (ALL): Low levels of BCR-ABL fusion transcripts in plasma can indicate residual disease post-treatment.
- Chronic lymphocytic leukemia (CLL): ctDNA tracking can identify relapse months before symptoms emerge.
- Breast and Colon Cancer:
- ctDNA monitoring post-surgery or chemotherapy helps detect micrometastatic disease, guiding decisions on adjuvant therapy.
- Example:
- Colorectal cancer: Studies show that patients with detectable ctDNA post-surgery have a significantly higher risk of relapse, justifying early intervention.
- Lung Cancer:
- Plasma-based MRD testing allows for early intervention in lung cancer recurrence, reducing reliance on imaging, which may miss small lesions.
- Example:
- NSCLC patients treated with surgery or radiation: Persistent ctDNA in plasma post-treatment suggests a need for additional therapy or close monitoring.
These studies highlight how longitudinal plasma analysis provides a highly sensitive tool for early cancer detection and MRD monitoring, enabling timely therapeutic interventions and improving survival rates.
Clinical Trial Applications
Longitudinal plasma is playing an increasingly critical role in oncology clinical trials, helping researchers develop more effective therapies while reducing trial costs and patient burden.
Key Benefits of Longitudinal Plasma in Clinical Trials:
- Identifying Patients Who Will Benefit from Targeted Therapies
- By using plasma biomarkers to pre-screen patients, clinical trials can enrich their cohorts with individuals most likely to respond to investigational drugs, improving trial success rates.
- Tracking Response Rates & Resistance Mechanisms
- Longitudinal plasma enables real-time monitoring of tumor response, allowing researchers to adjust trial protocols based on patient responses.
- Reducing Costs & Minimizing the Need for Repeated Biopsies
- Tissue biopsies in clinical trials can be costly, invasive, and impractical, especially in late-stage cancers. Plasma-based monitoring offers a more feasible, patient-friendly alternative.
Example: Plasma Biomarker-Based Clinical Trials
- LUNG-MAP (Lung Cancer Master Protocol): Uses plasma ctDNA analysis to match NSCLC patients with targeted therapies.
- BFAST Trial (Breast Cancer ctDNA Study): Uses longitudinal plasma to identify PIK3CA-mutated breast cancer patients for PI3K inhibitors.
By integrating plasma-based biomarker assessments, clinical trials can be optimized for greater efficiency, improved patient selection, and faster drug development.
The role of longitudinal plasma in oncology research is rapidly expanding, transforming biomarker discovery, personalized medicine, early cancer detection, and clinical trials. By enabling real-time, non-invasive tracking of tumor evolution, longitudinal plasma is paving the way for more precise, adaptive cancer treatments. As technological advances in liquid biopsy, NGS, and AI-driven analytics continue to evolve, the clinical applications of longitudinal plasma will only expand, making it an essential tool in the future of precision oncology.
Emerging Technologies & Analytical Approaches in Longitudinal Plasma Analysis
Technological advancements have significantly enhanced the sensitivity, specificity, and scalability of longitudinal plasma analysis in oncology. Traditional methods of cancer monitoring, such as tissue biopsies and imaging, have inherent limitations, including invasiveness and the inability to provide real-time molecular insights. However, emerging technologies like liquid biopsy, next-generation sequencing (NGS), multi-omics approaches, and artificial intelligence (AI)-driven analytics are revolutionizing the field. These innovations enable early cancer detection, treatment monitoring, and precision medicine strategies, making longitudinal plasma an indispensable tool in oncology research and clinical care.
Liquid Biopsy & Next-Generation Sequencing (NGS)
The Role of Liquid Biopsy in Longitudinal Plasma Analysis
A liquid biopsy is a minimally invasive method that analyzes circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), extracellular vesicles (EVs), and proteins in plasma to assess tumor characteristics. Compared to tissue biopsies, liquid biopsies offer real-time monitoring of cancer progression and treatment response, enabling longitudinal tracking of tumor evolution.
Advantages of Liquid Biopsy in Oncology:
- Non-invasive and repeatable: Enables frequent monitoring without the need for invasive tissue biopsies.
- Early detection of treatment resistance: Identifies emerging mutations that confer resistance to targeted therapies.
- Comprehensive tumor profiling: Captures tumor heterogeneity, which may not be reflected in a single tissue biopsy.
- Enhanced patient compliance: Less discomfort and risk compared to surgical biopsies.
Advancements in Next-Generation Sequencing (NGS) for Plasma Analysis
NGS has significantly improved the sensitivity and specificity of longitudinal plasma analysis by allowing high-throughput sequencing of ctDNA, enabling the detection of rare mutations that may drive cancer progression or resistance.
Key NGS-Based Assays in Longitudinal Plasma Research:
- Targeted NGS Panels: Focus on specific oncogenic mutations (e.g., EGFR, KRAS, TP53).
- Whole-Exome Sequencing (WES): Identifies genome-wide mutations that may contribute to therapy resistance.
- Whole-Genome Sequencing (WGS): Offers a comprehensive genomic landscape of tumors but requires deep sequencing for sensitivity.
NGS allows for:
- Early detection of cancer recurrence by identifying residual ctDNA post-treatment.
- Tracking the emergence of resistance mutations before clinical progression occurs.
- Improved treatment stratification by matching patients to precision oncology therapies.
Digital PCR (dPCR) and BEAMing Assays: Enhancing Sensitivity for Low-Abundance Mutations
One of the biggest challenges in longitudinal plasma analysis is detecting low-abundance mutations in ctDNA, which may be present at extremely low concentrations (as low as 0.01%). To address this, high-sensitivity techniques such as digital PCR (dPCR) and BEAMing assays have been developed.
Digital PCR (dPCR)
dPCR offers ultra-sensitive mutation detection by partitioning a DNA sample into thousands of individual reactions, amplifying rare tumor mutations with greater accuracy than conventional PCR.
Clinical Applications of dPCR in Longitudinal Plasma:
- Detection of minimal residual disease (MRD) in leukemia, lung cancer, and breast cancer.
- Tracking resistance mutations in plasma to guide therapy adjustments.
- Monitoring ctDNA dynamics in patients undergoing targeted therapy or immunotherapy.
BEAMing (Beads, Emulsions, Amplification, and Magnetics) Assays
BEAMing technology combines digital PCR with flow cytometry to provide highly sensitive detection of single nucleotide variants (SNVs) and copy number variations (CNVs) in plasma.
Advantages of BEAMing Assays:
- Detects mutations at ultra-low frequencies (0.01% ctDNA in plasma).
- Useful for monitoring response to targeted therapies (e.g., KRAS, EGFR mutations).
- Identifies minimal residual disease (MRD) after curative treatments.
Both dPCR and BEAMing assays have become crucial tools for high-resolution monitoring of tumor dynamics, improving the clinical utility of longitudinal plasma analysis.
Multi-Omics Approaches: A Holistic View of Tumor Biology
Integrating Genomics, Proteomics, and Metabolomics
To achieve a comprehensive understanding of tumor biology, researchers are integrating multi-omics approaches that combine:
- Genomics: Sequencing ctDNA to identify driver mutations and resistance mechanisms.
- Proteomics: Analyzing plasma proteins to assess tumor microenvironment and immune response.
- Metabolomics: Detecting metabolic signatures in plasma to track cancer metabolism and drug resistance.
By combining these molecular layers, multi-omics approaches provide a more complete picture of tumor evolution, improving:
- Cancer biomarker discovery.
- Prediction of therapy response.
- Identification of novel drug targets.
Mass Spectrometry in Plasma-Based Proteomics
Recent advances in mass spectrometry (MS) have made it possible to detect tumor-associated proteins in plasma, opening new possibilities for early cancer detection and treatment response monitoring.
Applications of Plasma-Based Mass Spectrometry:
- Identifying protein signatures associated with tumor progression.
- Tracking immune response biomarkers in patients receiving checkpoint inhibitors.
- Discovering novel plasma biomarkers for liquid biopsy applications.
The integration of genomics, proteomics, and metabolomics will further enhance precision medicine by offering multi-dimensional insights into tumor biology.
Artificial Intelligence & Big Data in Longitudinal Plasma Studies
AI and machine learning (ML) are playing a transformative role in the interpretation of longitudinal plasma data, enabling more accurate biomarker discovery, disease prediction, and patient stratification.
AI-Driven Biomarker Discovery
AI algorithms are helping identify novel ctDNA, RNA, and protein biomarkers that may have been overlooked using traditional methods. These approaches enhance:
- Cancer classification and staging through pattern recognition in plasma samples.
- Prediction of therapy response by analyzing longitudinal molecular data.
Machine Learning for Multi-Modal Data Integration
One of the biggest challenges in longitudinal plasma research is the integration of vast multi-modal datasets. AI-driven machine learning models are helping:
- Analyze plasma ctDNA mutations alongside imaging and clinical data for more accurate prognosis.
- Stratify patients in clinical trials based on real-time biomarker trends.
- Develop predictive models for cancer recurrence using longitudinal plasma data.
Real-World Applications of AI in Longitudinal Plasma Research
- Case Study: AI-Based MRD Detection
- AI-driven ctDNA analysis has been shown to predict relapse up to 6 months before clinical detection in colorectal and lung cancer patients.
- Case Study: AI for Immunotherapy Response Prediction
- Machine learning models analyzing plasma cytokine levels have been used to predict which patients will benefit from immune checkpoint inhibitors.
As AI continues to evolve, it will further optimize the utility of longitudinal plasma analysis, making cancer diagnosis and treatment more precise, predictive, and personalized.
The emergence of liquid biopsy, NGS, multi-omics approaches, and AI-driven analytics is driving rapid advancements in longitudinal plasma research. These technologies enable real-time, high-resolution monitoring of tumor evolution, allowing for earlier detection, better treatment response assessment, and improved patient outcomes.
As analytical techniques continue to evolve, the integration of machine learning, multi-omics, and high-sensitivity sequencing will further enhance the clinical utility of longitudinal plasma, solidifying its role as a cornerstone of precision oncology.
Challenges and Limitations of Longitudinal Plasma Analysis
While longitudinal plasma analysis has emerged as a powerful tool in oncology, several technical, clinical, regulatory, and ethical challenges must be addressed before it can be fully integrated into routine medical practice. These challenges include standardization issues, sensitivity limitations, regulatory complexities, and data interpretation hurdles, all of which impact the widespread adoption and clinical utility of plasma-based diagnostics. Overcoming these obstacles is essential for maximizing the potential of longitudinal plasma in early cancer detection, treatment monitoring, and personalized medicine.
Technical Challenges in Longitudinal Plasma Analysis
1. Standardization of Sample Collection and Processing
One of the most significant technical hurdles in longitudinal plasma analysis is the lack of standardization in sample collection, processing, and storage, which can affect biomarker stability and data reproducibility. Since circulating tumor DNA (ctDNA), proteins, and metabolites in plasma can degrade rapidly, even small differences in sample handling can lead to false-negative results or variability in biomarker detection.
Key Factors Affecting Plasma Sample Integrity:
- Collection Method:
- The type of blood collection tube used (EDTA, Streck, or PAXgene tubes) can affect ctDNA yield and stability.
- Immediate processing vs. delayed processing can influence biomarker degradation.
- Storage Conditions:
- Plasma samples require strict temperature control (-80°C for long-term storage).
- Repeated freeze-thaw cycles can degrade ctDNA and proteins.
- Centrifugation & Isolation Protocols:
- Differences in centrifugation speed and duration impact the separation of plasma from cellular components.
- Incomplete removal of white blood cells can lead to contamination with genomic DNA, reducing assay specificity.
Solution: International efforts, such as the BloodPAC Consortium, are working to establish standardized protocols for plasma sample collection and processing to improve inter-study reproducibility and enhance the reliability of longitudinal plasma analyses.
2. Sensitivity and Specificity Limitations in Early-Stage Cancer Detection
While liquid biopsy and ctDNA analysis have demonstrated high sensitivity in advanced cancers, detecting low-abundance mutations in early-stage cancers remains a challenge due to low ctDNA concentrations in plasma.
Why Sensitivity is a Major Challenge?
- Early-stage tumors shed less ctDNA into circulation compared to late-stage or metastatic cancers.
- Some cancers, such as glioblastoma and renal cell carcinoma, release very low levels of ctDNA, making detection difficult.
- High background noise from non-tumor-derived DNA (e.g., leukocyte-derived cfDNA) can obscure tumor-specific signals.
Potential Solutions:
- Ultra-deep sequencing approaches (e.g., NGS with error suppression techniques) to enhance mutation detection.
- Digital PCR (dPCR) and BEAMing assays for higher sensitivity in detecting rare mutations.
- Combining ctDNA analysis with protein and metabolite biomarkers to improve overall detection rates.
By refining these techniques, longitudinal plasma analysis can become more reliable for detecting cancer at its earliest stages, improving early intervention strategies.
Clinical and Regulatory Hurdles
1. Complex Regulatory Pathways for Plasma-Based Diagnostics
For longitudinal plasma analysis to be widely adopted in clinical practice, regulatory agencies like the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) must approve plasma-based liquid biopsy tests as reliable diagnostic tools. However, the regulatory approval process is challenging due to several factors:
- Lack of standardized validation criteria: Different clinical studies report varying levels of sensitivity and specificity, making it difficult to establish universally accepted guidelines
- Uncertainty in clinical endpoints: While ctDNA detection correlates with tumor burden, its role as a standalone biomarker for treatment decisions is still being evaluated.
- Need for large-scale validation trials: Regulatory bodies require extensive clinical trial data before approving plasma-based assays for routine use.
Current Regulatory Efforts:
- The FDA’s Breakthrough Devices Program is expediting the review process for innovative liquid biopsy tests.
- The European Commission’s IVDR (In Vitro Diagnostic Regulation) is working to establish clear guidelines for plasma-based diagnostics.
Standardizing regulatory pathways will accelerate clinical adoption, making longitudinal plasma a key component of routine oncology care.
2. Challenges in Integrating Longitudinal Plasma Analysis into Clinical Practice
Even after regulatory approval, seamless integration of plasma-based testing into routine oncology workflows remains a challenge due to cost, accessibility, and physician adoption barriers.
Barriers to Clinical Integration:
- Cost Considerations:
- NGS-based plasma assays can cost $1,000–$5,000 per test, making them expensive for widespread use.
- Insurance reimbursement policies for liquid biopsy tests are still evolving, limiting accessibility for many patients.
- Physician Awareness & Adoption:
- Many oncologists still rely on traditional tissue biopsies and may be unfamiliar with the clinical utility of longitudinal plasma analysis.
- Training programs for clinicians on how to interpret plasma-based biomarkers are needed.
- Need for Clear Clinical Guidelines:
- Oncologists need standardized protocols on how to integrate plasma-based testing into treatment decision-making.
- Consensus guidelines from ASCO (American Society of Clinical Oncology) and NCCN (National Comprehensive Cancer Network) are essential to ensure uniform adoption.
By addressing these integration challenges, longitudinal plasma testing can become a routine tool for real-time cancer monitoring.
Data Interpretation and Integration Challenges
1. Harmonizing Data Across Different Studies and Platforms
With the growing use of longitudinal plasma biomarkers, researchers and clinicians face challenges in standardizing data analysis methods across different platforms.
Key Issues in Data Harmonization:
- Variability in sequencing technologies (Illumina vs. Thermo Fisher vs. Oxford Nanopore) affects mutation detection rates.
- Different bioinformatics pipelines may yield conflicting interpretations of plasma-derived ctDNA data.
- Lack of centralized databases limits cross-study comparisons and validation of plasma-based biomarkers.
Potential Solutions:
- Creation of global databases to collect and compare longitudinal plasma biomarker data.
- Development of AI-driven data harmonization tools to standardize bioinformatics pipelines.
2. Ethical Concerns: Patient Privacy and Plasma-Based Genetic Data
With plasma-based sequencing becoming more prevalent, ethical concerns around patient privacy, consent, and genetic data security must be addressed.
Major Ethical Concerns:
- Genomic data security: Plasma-based tests generate highly sensitive genetic data, which, if misused, could pose privacy risks.
- Informed consent challenges: Patients may not fully understand how their plasma-derived genetic information will be stored, shared, or analyzed.
- Discrimination risks: Plasma-based genetic screening could raise concerns about insurance and employment discrimination based on cancer risk predictions.
Proposed Ethical Safeguards:
- Implementation of stronger data encryption and anonymization protocols for plasma-based sequencing data.
- Clear informed consent frameworks to ensure patients understand how their plasma-derived information will be used.
- Legislative protections, such as expanding GINA (Genetic Information Nondiscrimination Act) to include plasma-based diagnostics.
By proactively addressing data privacy and ethical issues, researchers can build public trust and promote ethical adoption of longitudinal plasma analysis.
While longitudinal plasma analysis holds tremendous potential for revolutionizing cancer diagnostics, treatment monitoring, and precision medicine, several technical, regulatory, and ethical challenges must be overcome for widespread clinical adoption.
- Standardizing plasma sample collection and processing is crucial for improving biomarker reliability.
- Enhancing the sensitivity of ctDNA detection will allow for earlier cancer detection and better treatment monitoring.
- Regulatory agencies must establish clear approval pathways for plasma-based diagnostics.
- Ethical frameworks must be strengthened to protect patient privacy and prevent genetic discrimination.
By addressing these challenges, longitudinal plasma analysis can achieve its full potential, paving the way for a new era of precision oncology.
Future Perspectives & Implications for Oncology Research
Longitudinal plasma is set to become a cornerstone of precision oncology, enabling non-invasive, real-time cancer monitoring. Emerging advancements in liquid biopsy, AI-driven analytics, immunotherapy monitoring, and industry collaboration are accelerating its clinical adoption.
1. Universal Liquid Biopsy Screening for Early Cancer Detection
Current liquid biopsies are mainly used for treatment monitoring, but next-generation sequencing (NGS) and ultra-sensitive ctDNA assays are driving the development of multi-cancer early detection (MCED) tests.
Key Benefits:
- Early detection before symptoms appear: Improved survival rates.
- Non-invasive and easily accessible: Blood tests replace tissue biopsies.
- Multi-cancer detection: A single test screens for multiple tumor types.
Example: Galleri™ Test
- Uses ctDNA methylation patterns to detect 50+ cancers in early stages.
- Still requires large-scale validation for widespread adoption.
Future Direction: AI-powered plasma screening integrated into routine cancer prevention programs.
2. AI-Driven Models for Personalized Therapy
Artificial intelligence is transforming plasma biomarker analysis, improving predictive modeling for treatment responses.
How AI Enhances Longitudinal Plasma Analysis:
- Identifies emerging resistance mutations: Enables proactive therapy adjustments.
- Stratifies patients for immunotherapy Predicts who will benefit from checkpoint inhibitors.
- Integrates multi-omics data Connects ctDNA, protein, and metabolite signatures for a full tumor profile.
Example: AI-Driven MRD Detection
- Predicts cancer relapse up to 6 months in advance via ctDNA fluctuations.
Future Direction: AI-assisted plasma diagnostics integrated into clinical decision-support tools.
3. Expanded Applications in Immunotherapy & CAR-T Monitoring
Longitudinal plasma is improving real-time tracking of immune response and CAR-T therapy efficacy.
Key Applications:
- ctDNA clearance as an immunotherapy response predictor: Faster therapy adjustments.
- Cytokine monitoring in plasma: Detects immune-related adverse events (irAEs) early.
- CAR-T relapse detection: Plasma biomarkers signal disease recurrence before symptoms arise.
Example: ctDNA Tracking in Checkpoint Inhibitors
- Persistent ctDNA post-treatment suggests therapy resistance, prompting alternative strategies.
Future Direction: Standardized immune biomarkers for therapy optimization.
4. Collaboration Between Academia, Biotech & Pharma
Accelerating clinical adoption requires global research partnerships between academia, biotech, and pharmaceutical companies.
Why It Matters:
- Faster clinical validation of plasma biomarkers.
- Better patient selection for drug trials using liquid biopsy insights.
- AI + Biotech integration → Advanced predictive plasma-based tools.
Recent Initiatives:
- BloodPAC Consortium: Standardizing liquid biopsy methodologies.
- Cancer Moonshot Initiative: Investing in ctDNA-based cancer detection.
- Future Direction: Creation of global plasma biomarker databases for cross-border research.
Longitudinal plasma is revolutionizing cancer detection, treatment monitoring, and precision medicine. Key future trends include:
- Universal plasma-based cancer screening.
- AI-driven therapy personalization.
- Advanced immunotherapy tracking.
- Stronger research collaborations.
With continuous technological advancements, longitudinal plasma will reshape oncology care, enabling earlier interventions and better patient outcomes.
With continuous technological advancements, longitudinal plasma will reshape oncology care, enabling earlier interventions and better patient outcomes.
Conclusion
Longitudinal plasma analysis is revolutionizing oncology research and clinical care by enabling real-time biomarker monitoring, personalized treatment adjustments, and early detection of minimal residual disease (MRD). Unlike traditional tissue biopsies, this non-invasive approach provides continuous insights into tumor evolution, allowing for earlier interventions and improved treatment strategies.
As technology advances, the integration of next-generation sequencing (NGS), AI-driven analytics, and multi-omics approaches will further enhance its clinical applications. Longitudinal plasma will play a key role in streamlining drug development, optimizing immunotherapy response monitoring, and improving early cancer detection, ultimately leading to better patient outcomes.
However, standardization, regulatory approvals, and data harmonization remain challenges that must be addressed for widespread adoption. Continued research, cross-disciplinary collaboration, and investment in liquid biopsy innovation are essential to unlocking the full potential of longitudinal plasma in precision medicine. With ongoing advancements, longitudinal plasma is poised to redefine cancer diagnostics, treatment, and long-term disease management, marking a new era in oncology.
FAQs