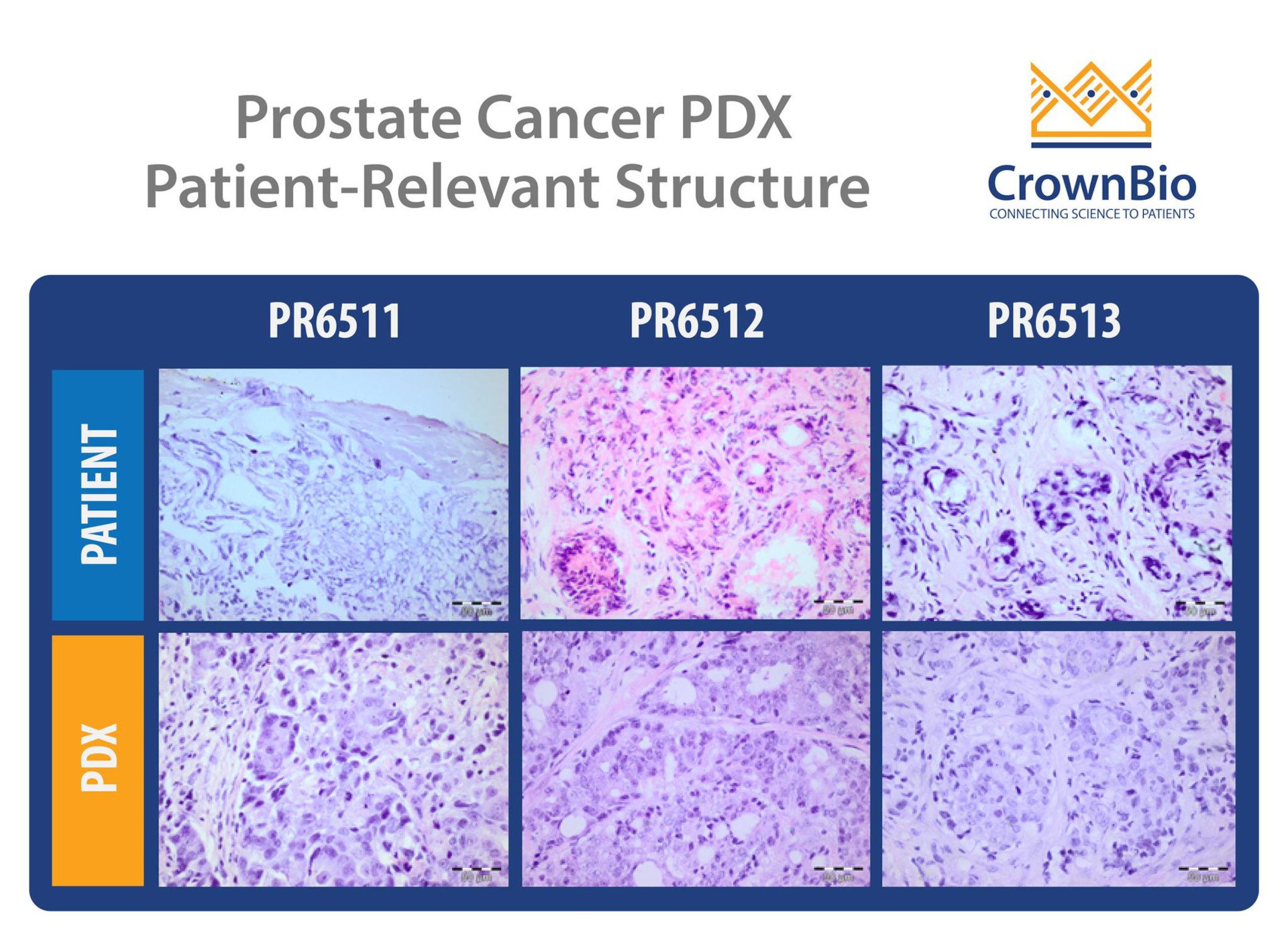
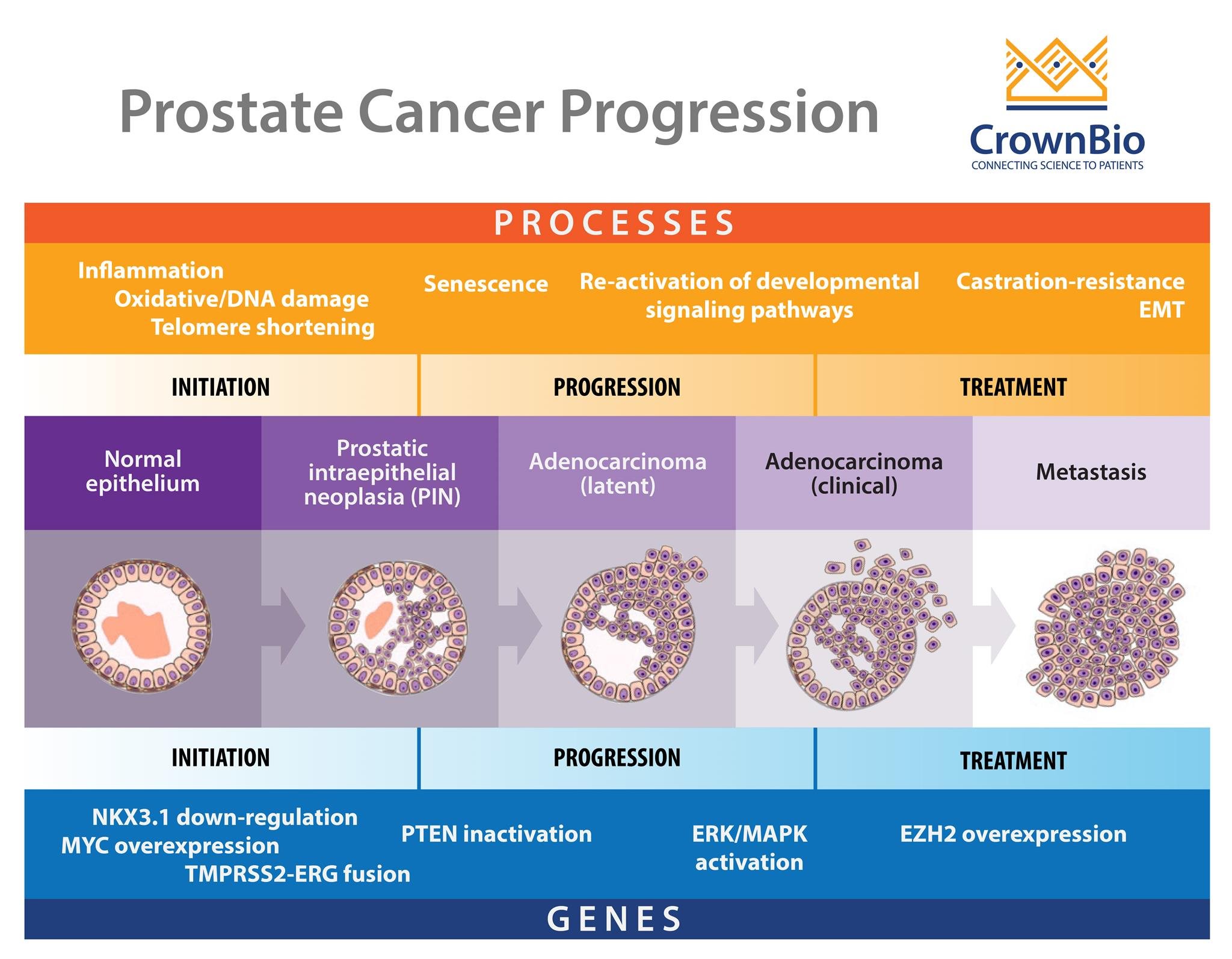
The integration of patient tumor samples and mass spectrometry is reshaping the landscape of cancer research and precision medicine. As researchers and clinicians seek to understand the complexities of cancer at a molecular level, high-resolution analytical techniques have become essential. Mass spectrometry (MS), when applied to patient-derived tumor tissues, allows for comprehensive profiling of proteins, lipids, metabolites, and post-translational modifications—offering insights into tumor biology, progression, and therapeutic vulnerabilities.
This article explores how mass spectrometry, applied to patient tumor samples, is advancing biomarker discovery, therapeutic targeting, and individualized treatment strategies across cancer types.
Unlike traditional histological or genetic analyses, mass spectrometry can directly quantify functional molecules within the tumor microenvironment, offering a real-time snapshot of the tumor’s metabolic and proteomic state. This enables researchers to capture dynamic cellular changes, such as treatment-induced modifications or evolving resistance mechanisms, with a high degree of specificity and sensitivity. Such detailed molecular phenotyping is critical for the development of next-generation therapies and diagnostics.
Moreover, mass spectrometry is uniquely positioned to bridge the translational gap between bench and bedside. When used in conjunction with patient-derived xenografts, organoids, or fresh-frozen biopsy samples, MS technologies can identify actionable biomarkers and drug targets that are difficult to detect through genomic analysis alone. As a result, it plays a pivotal role in the advancement of truly personalized cancer treatments, ensuring that therapeutic strategies are guided not only by genetic mutations but also by the functional state of the tumor.
The Promise of Precision Oncology
Precision oncology aims to tailor treatment based on the unique molecular characteristics of a patient's tumor. While genomic sequencing has been the cornerstone of this approach, it only tells part of the story. Tumor proteomics and metabolomics, analyzed via mass spectrometry, provide functional insights that bridge the gap between genotype and phenotype.
Mass spectrometry enables:
- Quantitative proteomics: Mapping protein expression and abundance.
- Phosphoproteomics: Identifying signaling pathway dysregulations.
- Lipidomics and metabolomics: Understanding energy metabolism and tumor microenvironment.
- Biomarker validation: Detecting novel cancer-specific molecular signatures.
The ability to analyze proteins and metabolites at a systems level provides a more holistic view of tumor biology. For instance, post-translational modifications—such as phosphorylation or acetylation—can drastically alter protein function and are often missed by DNA- or RNA-based approaches. Mass spectrometry is uniquely capable of capturing these changes, revealing how cancer cells adapt, communicate, and resist therapeutic pressure. This layer of molecular data offers actionable insights into oncogenic pathways that drive tumor survival and progression.
Furthermore, mass spectrometry supports real-time monitoring of tumor evolution. By comparing longitudinal samples from the same patient before, during, and after treatment, clinicians can identify shifts in protein expression or metabolic states that signify emerging resistance or therapeutic efficacy. These insights not only enhance patient stratification in clinical trials but also inform adaptive treatment regimens in routine care—pushing the boundaries of truly dynamic, responsive cancer therapy.
Why Use Patient Tumor Samples?
Biological Relevance
Cell lines and animal models, while indispensable, often fail to capture the full heterogeneity of human tumors. Patient tumor samples—whether from biopsies, resections, or xenografts—preserve native tissue architecture, immune infiltration, and molecular diversity. These characteristics make them ideal for clinically relevant MS-based analyses.
Unlike immortalized cell lines that adapt to artificial culture conditions, patient-derived tissues retain the heterogeneity seen in real tumors, including subclonal populations, variable expression profiles, and unique mutational burdens. This complexity is essential for understanding differential drug responses and resistance mechanisms that may not emerge in more homogeneous models. By studying these variations directly in patient samples, researchers can uncover rare but clinically significant molecular features that drive treatment outcomes.
Additionally, patient tumor samples provide the opportunity to study disease evolution in a longitudinal manner. Serial biopsies taken over the course of treatment allow mass spectrometry to track molecular alterations in response to therapy or disease progression. This temporal resolution helps identify dynamic biomarkers, transient resistance pathways, and novel intervention points that are missed in static, one-time model systems.
Tumor Microenvironment Insights
Mass spectrometry of patient samples can reveal the composition and interactions within the tumor microenvironment (TME), including immune cells, stromal components, and extracellular matrix proteins. These findings are crucial for immuno-oncology research and the development of combination therapies.
TheTME plays a decisive role in tumor progression, metastasis, and immune evasion. Using spatially resolved mass spectrometry, such as MALDI imaging, researchers can map protein and metabolite gradients across tumor boundaries, distinguishing invasive edges from necrotic cores or immune-rich zones. These insights inform the development of localized therapies and guide the selection of immune-modulating agents based on microenvironmental composition.
Moreover, MS-based proteomic profiling can identify biomarkers of immune activation or suppression—such as checkpoint ligand expression, cytokine signaling pathways, or metabolic reprogramming of immune cells within the TME. This level of detail is essential for designing personalized immunotherapy combinations, evaluating the likelihood of response to checkpoint inhibitors, and developing predictive companion diagnostics. As immuno-oncology continues to expand, understanding the dynamic crosstalk between tumor cells and their microenvironment through patient samples becomes ever more critical.
Mass Spectrometry Technologies in Cancer Research
Several MS-based approaches are widely used to analyze patient tumor samples, each offering distinct advantages depending on the research or clinical objective.
1. Liquid Chromatography-Mass Spectrometry (LC-MS/MS)
LC-MS/MS is the gold standard for deep proteomic analysis. It enables the high-resolution separation and identification of thousands of proteins from complex tumor tissues. By coupling liquid chromatography with tandem mass spectrometry, researchers can achieve both broad coverage and accurate quantification of proteins and peptides. Recent advances in data-independent acquisition (DIA), label-free quantification, and tandem mass tags (TMT) have significantly improved the sensitivity, reproducibility, and throughput of LC-MS/MS.
This technology is particularly well-suited for discovering differentially expressed proteins in patient tumors, which may serve as diagnostic markers or therapeutic targets. In longitudinal studies, LC-MS/MS has been used to monitor proteomic shifts in response to treatment, helping identify early indicators of resistance or treatment efficacy. Its versatility also supports phosphoproteomics, enabling pathway-specific analyses that reveal dysregulated signaling cascades in cancers such as lung, colorectal, and breast tumors.
2. Matrix-Assisted Laser Desorption/Ionization (MALDI) Imaging
MALDI-MS provides spatially resolved molecular imaging directly on tissue sections without the need for antibodies or staining. This label-free method generates ion maps of proteins, lipids, and metabolites, which can be overlaid on histological images to visualize the distribution of molecular species across distinct tumor regions. MALDI imaging is particularly powerful for studying tumor heterogeneity, invasive margins, and the interaction between cancerous and non-cancerous cells.
For example, MALDI-MS has been employed to distinguish between low-grade and high-grade gliomas based on spatially distinct protein signatures. It also aids in identifying biomarkers of drug resistance that localize to specific tumor niches. Beyond proteomics, the technique can be adapted for lipidomics and metabolomics, making it a multidimensional tool for tumor characterization. Its integration with pathology workflows is also opening new possibilities for intraoperative diagnostics and targeted surgical resection.
3. Targeted MS: Multiple Reaction Monitoring (MRM)
MRM is a targeted mass spectrometry approach used for the accurate quantification of predefined panels of proteins or peptides. Unlike discovery proteomics, which aims to identify as many proteins as possible, MRM focuses on reproducibly measuring specific biomarkers with high sensitivity and precision. It is particularly valuable for validating candidate biomarkers identified through LC-MS/MS and for clinical applications requiring standardized, high-throughput analysis.
MRM has been successfully implemented in studies quantifying proteins associated with breast cancer subtypes, treatment resistance markers in prostate cancer, and inflammatory mediators in the tumor microenvironment. Its robustness makes it ideal for longitudinal monitoring of patient samples, including serum or plasma, and it is increasingly being adopted in clinical trials as a companion diagnostic tool. Moreover, recent developments in parallel reaction monitoring (PRM) and data integration tools are enhancing its scalability and adaptability to multiplexed biomarker panels.
Key Applications in Precision Medicine
Biomarker Discovery and Validation
Mass spectrometry has revealed novel biomarkers associated with cancer progression, therapeutic response, and resistance. For example, differential expression of phosphoproteins in lung cancer or specific metabolite profiles in glioblastoma can serve as actionable biomarkers.
MS-based proteomics and metabolomics provide the depth and resolution necessary to detect subtle yet clinically significant molecular changes that might be missed by traditional assays. This capability is especially crucial in early-stage cancers or in minimal residual disease settings, where early detection can drastically improve outcomes. Furthermore, MS allows researchers to identify multi-analyte biomarker panels that combine proteins, lipids, and metabolites—offering a more holistic view of tumor state and increasing diagnostic accuracy.
The reproducibility of mass spectrometry also makes it ideal for validating biomarkers across large patient cohorts. Once candidate markers are identified in discovery studies, they can be confirmed and quantified in independent samples using targeted MS approaches like MRM or PRM. These workflows are essential for transitioning biomarkers from research to clinical use and for ensuring that they maintain performance across diverse populations and tumor subtypes.
Therapeutic Target Identification
MS can identify overexpressed or mutated proteins that are amenable to drug targeting. For instance, analysis of HER2+ breast cancer tissues via MS confirmed proteomic overexpression patterns correlating with treatment response to trastuzumab.
This functional insight goes beyond genomics by highlighting post-translational modifications or protein isoforms that influence drug sensitivity. For example, proteomic profiling of colorectal tumors has revealed EGFR signaling variants that are not apparent at the DNA level but are essential for predicting response to anti-EGFR therapies. MS is particularly adept at identifying aberrant kinases, transcription factors, and cell surface proteins that serve as potential drug targets.
In drug development, these insights inform the rational design of targeted therapies. Patient tumor samples can be screened for expression of druggable targets, enabling early identification of patient subgroups who are likely to benefit from novel compounds. This reduces trial-and-error prescribing and accelerates the path from bench to bedside for emerging therapeutics.
Drug Mechanism and Resistance Profiling
By analyzing pre- and post-treatment patient samples, mass spectrometry can track the downstream effects of therapy at a molecular level, identifying resistance mechanisms and suggesting alternate treatment strategies.
Resistance to cancer therapy remains one of the greatest challenges in oncology. Tumors often evolve to bypass targeted pathways, upregulate compensatory mechanisms, or alter their microenvironment. MS enables detailed tracking of these adaptations by monitoring shifts in protein expression, phosphorylation status, and metabolic pathways. For example, phosphoproteomic analysis of melanoma samples has identified alternative kinase activations following BRAF inhibitor treatment—highlighting new intervention points.
Furthermore, MS can detect early molecular signs of relapse even before clinical symptoms emerge. By monitoring longitudinal tumor samples or even circulating tumor material (e.g., exosomes or plasma proteins), clinicians can proactively adjust treatment strategies to delay or prevent resistance. This capacity supports the development of adaptive therapeutic regimens, a cornerstone of personalized oncology.
Companion Diagnostics
Integrating MS data into diagnostic workflows can guide therapy selection. Patient stratification based on proteomic signatures ensures that patients receive the most effective treatments with minimal toxicity.
Mass spectrometry-based companion diagnostics are emerging as powerful tools for selecting appropriate therapies, particularly in cases where genomic markers are absent or ambiguous. For example, MS can quantify protein-level expression of receptors like HER2, PD-L1, or MET with greater accuracy than traditional immunohistochemistry. This provides a more precise assessment of targetable pathways and informs the use of biologics, immune checkpoint inhibitors, or kinase inhibitors.
As healthcare shifts toward value-based care, the ability to match treatments with patients likely to benefit becomes increasingly important. MS-driven companion diagnostics enhance this precision and help avoid unnecessary toxicity and cost. Moreover, the high-throughput nature of targeted MS allows for multiplexed testing of multiple biomarkers in a single run, improving efficiency and supporting the integration of personalized medicine into routine clinical workflows.
Integrative Omics: Mass Spectrometry Meets Genomics
The synergy between proteomics, genomics, and transcriptomics is crucial for a holistic understanding of cancer biology. Integrative analyses using patient tumor samples can:
- Validate gene expression at the protein level
- Identify non-genetic drivers of tumor behavior
- Predict functional consequences of mutations
This multi-omics integration is a cornerstone of systems oncology and is pivotal in biomarker-driven clinical trials.
While genomic and transcriptomic profiling identify mutations, copy number alterations, and gene expression changes, they often fall short of explaining actual cellular function. Protein activity is regulated not only by gene expression but also by post-translational modifications, localization, and interactions—features that are detectable only through proteomics. Mass spectrometry fills this gap by confirming whether a mutated gene leads to altered protein function, overexpression, or pathway activation. This allows researchers to discern which mutations are truly oncogenic drivers and which are biologically silent passengers.
Moreover, combining mass spectrometry with transcriptomics and genomics enhances predictive modeling for patient stratification and therapy selection. For instance, tumors with similar genetic profiles may exhibit vastly different proteomic signatures, leading to divergent clinical outcomes. Integrative omics enables the classification of tumors into more precise molecular subtypes, each with tailored therapeutic vulnerabilities. This convergence of data not only advances our understanding of tumor heterogeneity but also underpins the design of more effective, personalized treatment strategies and the identification of robust, multi-dimensional biomarkers for clinical decision-making.
Challenges and Future Directions
Technical Challenges
- Sample heterogeneity: Requires meticulous sampling and normalization.
- Low-abundance proteins: May be undetectable without enrichment techniques.
- Formalin-fixed, paraffin-embedded (FFPE) samples: Often require optimized protocols due to protein cross-linking.
One of the most pressing challenges in using patient tumor samples is biological heterogeneity. Tumors often contain multiple subclones and diverse microenvironmental components, including immune and stromal cells. Inconsistent sampling from different tumor regions can result in significant variability in MS data. This necessitates careful experimental design, standardized tissue processing, and computational normalization strategies to ensure reproducibility and accurate interpretation of results.
Moreover, many clinically relevant biomarkers, such as cytokines, signaling proteins, and transcription factors, are expressed at low levels and may fall below the detection threshold of standard MS workflows. Enrichment strategies, such as immunoprecipitation or fractionation, are often required but can be labor-intensive and introduce additional variability. FFPE samples, the most common form of archived clinical material, pose additional difficulties due to protein cross-linking during fixation. Specialized extraction and digestion protocols are needed to retrieve usable proteomic data, and even then, coverage may be limited compared to fresh-frozen samples.
Clinical Translation
To fully realize the clinical potential of mass spectrometry:
- Standardization across labs and platforms is essential.
- Regulatory approval of MS-based diagnostics must be streamlined.
- Data integration with electronic health records and AI-driven analytics will facilitate personalized care.
The transition of mass spectrometry from a research tool to a clinical mainstay hinges on the development of robust, standardized workflows. Currently, variability in sample preparation, instrument calibration, and data processing limits reproducibility across laboratories. International efforts such as the Clinical Proteomic Tumor Analysis Consortium (CPTAC) are working to address these challenges, but widespread adoption of best practices is still needed for clinical implementation.
Regulatory approval of MS-based diagnostics also remains a bottleneck. Unlike PCR or immunohistochemistry, mass spectrometry assays are more complex and require comprehensive validation to meet clinical lab standards (e.g., CLIA, CAP). Collaborations between regulatory bodies, academic researchers, and commercial developers are needed to streamline this process. Furthermore, to maximize clinical utility, MS data must be seamlessly integrated into electronic medical records and analyzed using AI-driven platforms that can translate molecular signatures into actionable clinical insights in real time.
Emerging Trends
- Single-cell proteomics: Capturing intratumoral heterogeneity at an unprecedented resolution.
- Spatial multi-omics: Combining MS imaging with RNA-seq and spatial transcriptomics.
- Artificial intelligence (AI): Enhancing pattern recognition and predictive modeling from complex MS datasets.
Single-cell proteomics is poised to transform cancer biology by enabling the profiling of individual cells within heterogeneous tumor populations. Recent innovations in sample preparation, nanoLC-MS, and ultra-sensitive detection are making it possible to quantify hundreds of proteins in single cells. This approach provides unprecedented insight into cellular diversity, revealing rare cell populations, lineage trajectories, and niche-specific signaling that influence tumor behavior and therapeutic response.
Spatial multi-omics is another frontier, combining mass spectrometry imaging with spatial transcriptomics and RNA sequencing to map molecular landscapes within tumor tissues. This integration allows researchers to pinpoint where specific proteins, transcripts, and metabolic changes occur within the tumor microenvironment, creating a multi-dimensional atlas of cancer biology. Coupled with AI and machine learning, these data-rich platforms enable the discovery of complex patterns, prediction of treatment response, and real-time stratification of patients. These emerging trends are not only expanding the analytical power of mass spectrometry but also bringing us closer to a future of truly personalized and spatially-informed oncology.
Conclusion
The combination of patient tumor samples and mass spectrometry is revolutionizing cancer research and personalized medicine. From uncovering hidden molecular signatures to guiding treatment decisions, this integrative approach is enhancing our understanding of cancer’s complexity and heterogeneity. Mass spectrometry provides the molecular resolution needed to detect subtle yet critical changes in tumor biology, empowering clinicians and researchers to move beyond one-size-fits-all treatments.
As MS technologies evolve and integrate with other omics platforms, their application in clinical oncology will only deepen—moving us closer to a future where every patient’s treatment is guided by precise molecular intelligence. The convergence of proteomics, genomics, metabolomics, and AI-driven analytics enables a systems-level view of cancer that is both actionable and scalable. This vision aligns with the goals of precision medicine: not just to treat cancer, but to anticipate, prevent, and outmaneuver it at the molecular level.
Looking ahead, the success of mass spectrometry in clinical practice will depend on continued investment in standardization, data integration, and interdisciplinary collaboration. Advances in single-cell analysis, spatial resolution, and real-time biomarker monitoring are already pushing the boundaries of what is possible. With patient tumor samples as the cornerstone, mass spectrometry is not just a tool for discovery—it is a transformative force in the quest to make cancer care more personalized, predictive, and effective.
FAQs