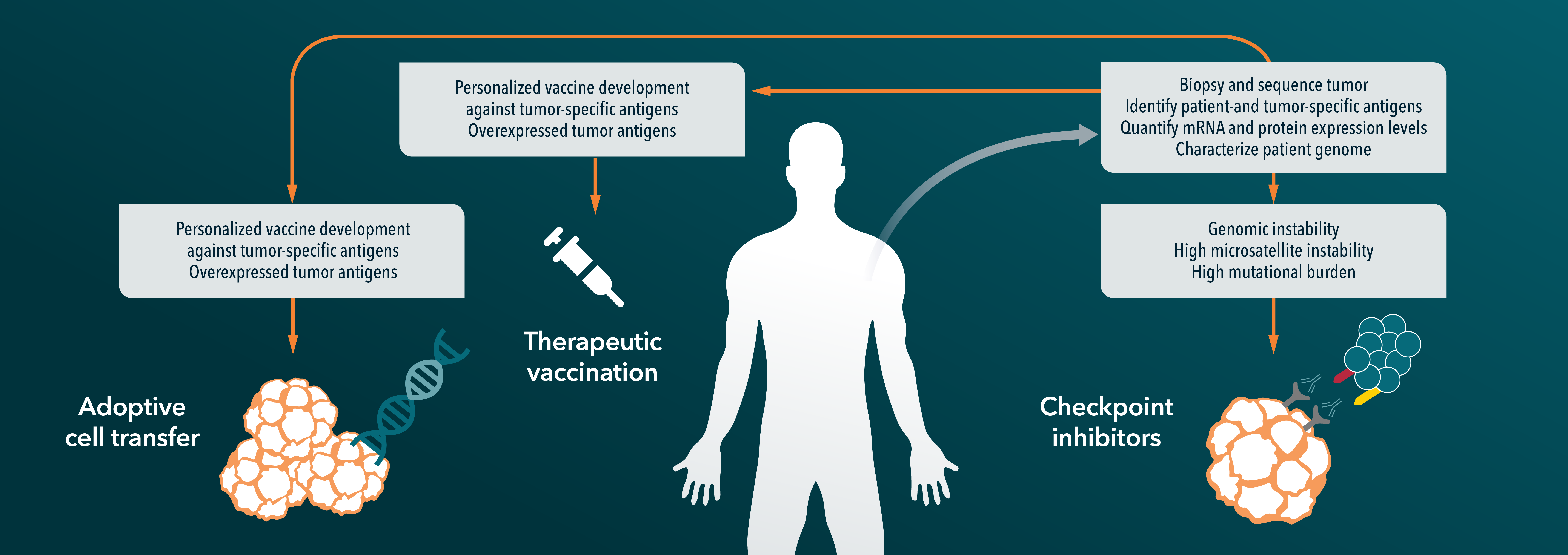
Precision medicine and immunotherapy continue to top the list as two of the hottest areas in cancer research. In this blog post we explore how immuno-oncology can draw from precision medicine to create more personalized cancer therapies to enhance their safety and efficacy. Read on to review how ICIs, neoantigen vaccines and adoptive cell therapies, including CAR-T, CAR-NK, CAR-M, TCR-T and TIL therapies, are advancing this remarkable field of science and medicine.
Why Personalized Precision Immunotherapy?
Precision medicine refers to the tailoring of medical treatments to the individual characteristics of a patient. This therapeutic customization involves identifying which approaches will be the most effective based on genetic, environmental, and lifestyle factors.
Immunotherapy aims to stimulate (or restore) a patient’s own immune system to fight cancer. Immune checkpoint blockade via anti-PD-1, PD-L1, and CTLA-4 inhibitors has demonstrated that the immune system is a critical player in combating cancer, and that immunotherapies are a key new treatment class. Immune checkpoint inhibitors (ICIs) are currently being used with great success to treat a variety of solid and hematologic malignancies at different stages.
However, only a fraction of patients develop a robust response to ICIs and patients can also experience significant side effects. Further, it is well-known that individual tumor characteristics—such as phenotypic, genetic, and the tumor microenvironment—all contribute to different patient responses, even within the same indication.
Therefore, there is an immediate need for improved immunotherapies that are tailored to the individual characteristics of each patient using a biomarker-based approach that relies on specific phenotypic, genetic, and/or tumor microenvironment features.
To this point, sponsors should consider creating a biomarker strategy early in drug development. This offers the best opportunity for selecting optimal targets and preclinical models, which can increase the chances that preclinical biomarkers will translate to the clinic, to create more personalized cancer immunotherapies and improve patient outcomes. An early biomarker strategy also offers good lead time required to develop companion diagnostics.
Below we discuss potential approaches for improving precision immunotherapies across the following therapeutic categories:
1. ICI therapies
2. Neoantigen vaccines
3. Adoptive cell therapy (ACT).
Immune-Checkpoint Inhibitor (ICI) Therapy
ICIs are the most extensively used immunotherapy for treating cancer but despite substantial success in some patients, the majority do not respond or are resistant to ICI therapy.
Currently, there are three FDA-approved biomarkers to help predict ICI response:
- Programmed cell death ligand-1 (PD-L1);
- Microsatellite instability (MSI); and
- Tumor mutational burden (TMB).
However, these biomarkers have been found to have minimal overlap, and therefore, they likely capture unique contributing factors to ICI response. There is a critical need for novel predictive biomarkers to match the right patients to the right ICI treatments. Several novel gene signature biomarkers are currently under development, including:
- T cell inflamed gene expression profile (GEP),
- T cell dysfunction and exclusion gene signature (TIDE),
- Melanocytic plasticity signature (MPS),
- B cell focused gene signature, and
- Combinations of predictive biomarkers (e.g., TMB+GEP; MPS+TIDE).
In addition to improve predictive biomarkers, combination therapies that increase neoantigen-specific T cell responses represent another method through which ICI therapy can be improved.
For instance, radiotherapy and/or chemotherapy are capable of increasing the number of neoantigens. When combined with ICI treatment, this enhances tumor-fighting neoantigen-specific T cells, and an increase in both the magnitude and specificity of the antitumor immune response can be achieved. Patients can also be vaccinated with their own tumor cells to enhance the number of neoantigens available for T cells to respond to.
Neoantigen Vaccines
Cancer is a mutation-driven disease that can alter a tumor’s antigen status to generate:
• Tumor-associated antigens (TAAs) - these arise from over- or aberrant expression of non-mutated proteins. TAAs have elevated levels on tumor cells but they are also expressed at lower levels in healthy cells.
• Tumor-specific antigens (TSAs) - also referred to as “neoantigens,” these are caused by mutations that are highly-tumor specific and they are abundantly expressed in/on cancer cells and have strong immunogenicity. Neoantigens arise from non-synonymous somatic variations that are randomly acquired during cell division.
Studies have shown that tumors with a high mutational burden, such as melanoma and lung cancer, are more likely to express immunogenic neoantigens. These neoantigens are not only unique to the tumor, but to the individual patient as well. In short, neoantigens are new antigens that a person’s immune system has not yet encountered, and as such, cells carrying neoantigens are targeted and destroyed.
Cancer immunotherapies are now being personalized by targeting patient-personalized immunogenic neoantigens. For instance, neoantigen vaccines are under development with the goal of eliciting de novo immune responses that enhance and broaden the endogenous repertoire of tumor-specific T cells that selectively target and kill cancer cells bearing these antigens, while leaving non-cancer cells untouched.
Another benefit offered by this approach is that the neoantigen-specific T cell responses may persist and provide long-term protection against disease recurrence. Relative to traditionally used TAA-based vaccines, neoantigen vaccines have several advantages including stronger immunogenicity and higher tumor specificity.
Cost-effective sequencing and bioinformatic technologies are now widely available to support the development and use of neoantigen vaccines, such as studies that have demonstrated robust tumor-specific immunogenicity, and preliminary anti-tumor effects in melanoma patients. The flow diagram below depicts the neoantigen synthesis process.
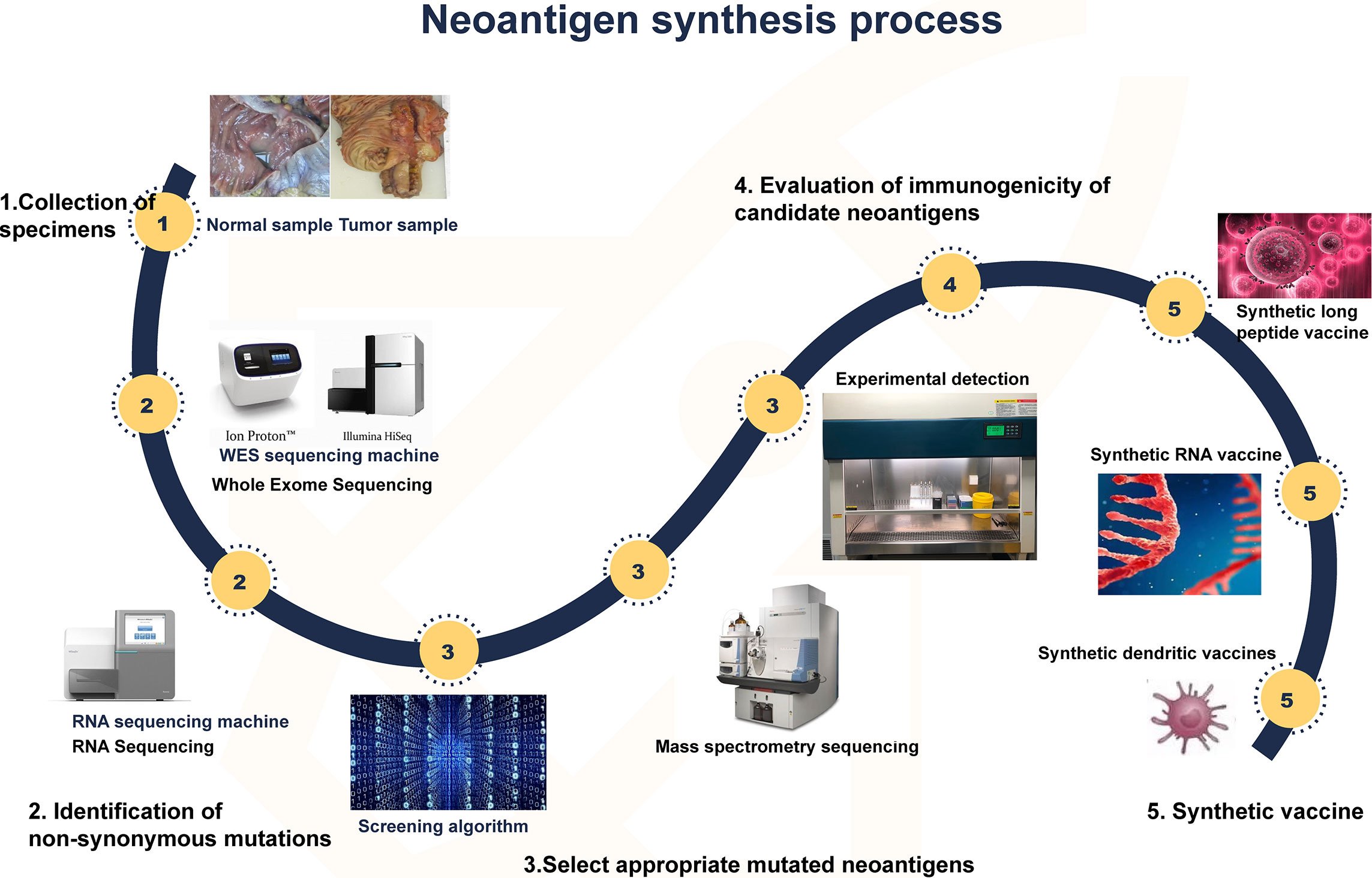
Synthesis of neoantigen. First, tumor and normal tissue samples were obtained. Then, by comparing the sequencing results of the two groups of samples, the mutated gene of the tumor was identified. Using computer, mass spectrometry or experimental methods to screen the gene sequences that are most likely to become tumor neoantigens, and finally these mutated genes can be designed into vaccines, which can take many forms, such as peptide vaccines, dendritic cell vaccines, RNA vaccines, etc. From Zhang et. al. (2021). Neoantigen: A New Breakthrough in Tumor Immunotherapy. Front. Immunol., 16 April 2021; doi:10.3389/fimmu.2021.672356. Used under Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Adoptive Cell Therapy (ACT)
ACTs are a rapidly evolving therapeutic approach that harness T cell specificity and function to attack tumor cells. Different types of ACTs include chimeric antigen receptor (CAR) T cell therapy, CAR-NK (natural killer)/CAR-M (macrophage) cell therapy, T cell antigen receptor (TCR) T cell therapy, and tumor-infiltrating lymphocyte (TIL) therapy.
While CAR-T, CAR-NK and CAR-M cells are designed to re-direct towards surface TAAs, TCR, and TIL therapies target human leukocyte antigen (HLA)-presented complexes. Each of these therapies are described below, including ways that these therapies may improve upon other technologies.
CAR-T Cell Therapies
One type of personalized immunotherapy that has already been approved for patient use is CAR-T cell therapy, a novel immuno-oncology treatment paradigm. CAR-T cells are autologous, or allogeneic (from a donor), T cells which specifically target tumor cell surface TAAs.
The first CAR-T therapy to be FDA-approved was Kymriah™ (tisagenlecleucel) in 2017, which targets the CD19 antigen - the most commonly targeted antigen with CAR-T cell therapy. Kymriah was approved for the treatment of patients up to 25 years old with relapsed and/or refractory B cell precursor acute lymphoblastic leukemia (ALL). Approval was based on a remarkable overall remission rate of 82.5%. The following table provides a listing of FDA-approved autologous CAR-T cell therapies, targeting CD19 or BCMA.
FDA Approved CAR-T Cell Therapies

*This table is from “CAR-T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers” and was originally published by the National Cancer Institute.
A few recognized limitations associated with CAR-T cell therapies include unique side effects and CAR-T cell exhaustion which can limit its efficacy, particularly in solid tumors. New CAR-T formats under development are using a variety of strategies to improve safety and counteract CAR-T cell exhaustion, including inhibition of exhaustion-promoting signaling, inhibition of downstream effectors, and modification of the CAR.
While the use of “off-the-shelf” allogeneic CAR-T cells from donors has several advantages over autologous CAR-T cells, including their immediate availability, lower cost, and no limitation of cell numbers, they come with some disadvantages including graft versus host disease (GvHD) and rapid elimination.
CAR-NK and CAR-M Therapies
Unlike T cells, NK cells can directly recognize target cells in the absence of MHC, and they can kill cancer cells through both CAR-dependent and CAR-independent pathways. Additional advantages of using CAR Natural Killer (NK) cells over CAR-T cells is that they can be manufactured from pre-existing cell lines or allogeneic NK cells with unmatched MHC, and they have better safety profiles, including a reduced risk of cytokine-release syndrome and neurotoxicity.
CAR-Macrophages (M) are also under active development. CAR-M cells take advantage of macrophages’ abilities to infiltrate into tumors, regulate the immune system, and their significant presence in the TME. CAR-M cells have edited CARs to target specific antigens for recognizing tumor cells and improving their phagocytic ability.
Furthermore, immunosuppressive M2 macrophages have strong phagocytic activity which is as efficient as that of proinflammatory M1 macrophages, and M2 macrophages can also be induced to differentiate to the M1 phenotype. Continued advances in CAR-M cells for cancer immunotherapy may help overcome some of the limitations associated with CAR-T/NK therapy, especially in solid tumors.
There are currently no FDA-approved drugs in this category, but many ongoing clinical trials worldwide.
TCR-T Therapies
T cell antigen receptor (TCR)-T cell therapy and CAR have similar but distinct signaling mechanisms that result in different persistence, toxicity, and therapeutic outcomes. TCR-T cell therapies are specific and sensitive for targeting cell-surface HLAs, and TCR-T cells can penetrate tumors, unlike CAR-T cells which are mainly distributed on the tumor periphery. Additional advantages of TCR-T cells versus CAR-T cells is that they have greater immunoreceptor tyrosine-based activation motifs (ITAMs), more subunits in their receptor structure, and less dependent on antigens.
There are currently no FDA-approved TCR-T cell therapies, but their efficacy and safety are being investigated in many ongoing clinical trials worldwide.
TIL Therapies
This type of cell therapy uses harvested lymphocytes that have infiltrated a tumor and are actively engaged in killing tumor cells. TIL therapy has several recognized benefits for treating solid tumors including diverse TCR clonality, superior tumor-homing ability, and low off-target toxicity.
TIL cell therapies have traditionally been generated using a tumor biopsy is used to identify neoantigens which are then inserted into autologous dendritic cells that are co-cultured with the TILs isolated from a tumor biopsy. TILs that recognize the neoantigen(s) are then selected, expanded, and infused back into the patient. Newer protocols (often referred to as the “Young TIL” approach) have been shown to have comparable clinical outcomes as the traditional method while circumventing the selection step which has shortened the timeline for TIL production as well as providing several other advantages. The two described approaches for generating TIL cell therapies (“traditional” and “young”) are illustrated below.
There are currently no FDA-approved TIL cell therapies, but their efficacy and safety are being investigated in many ongoing clinical trials worldwide.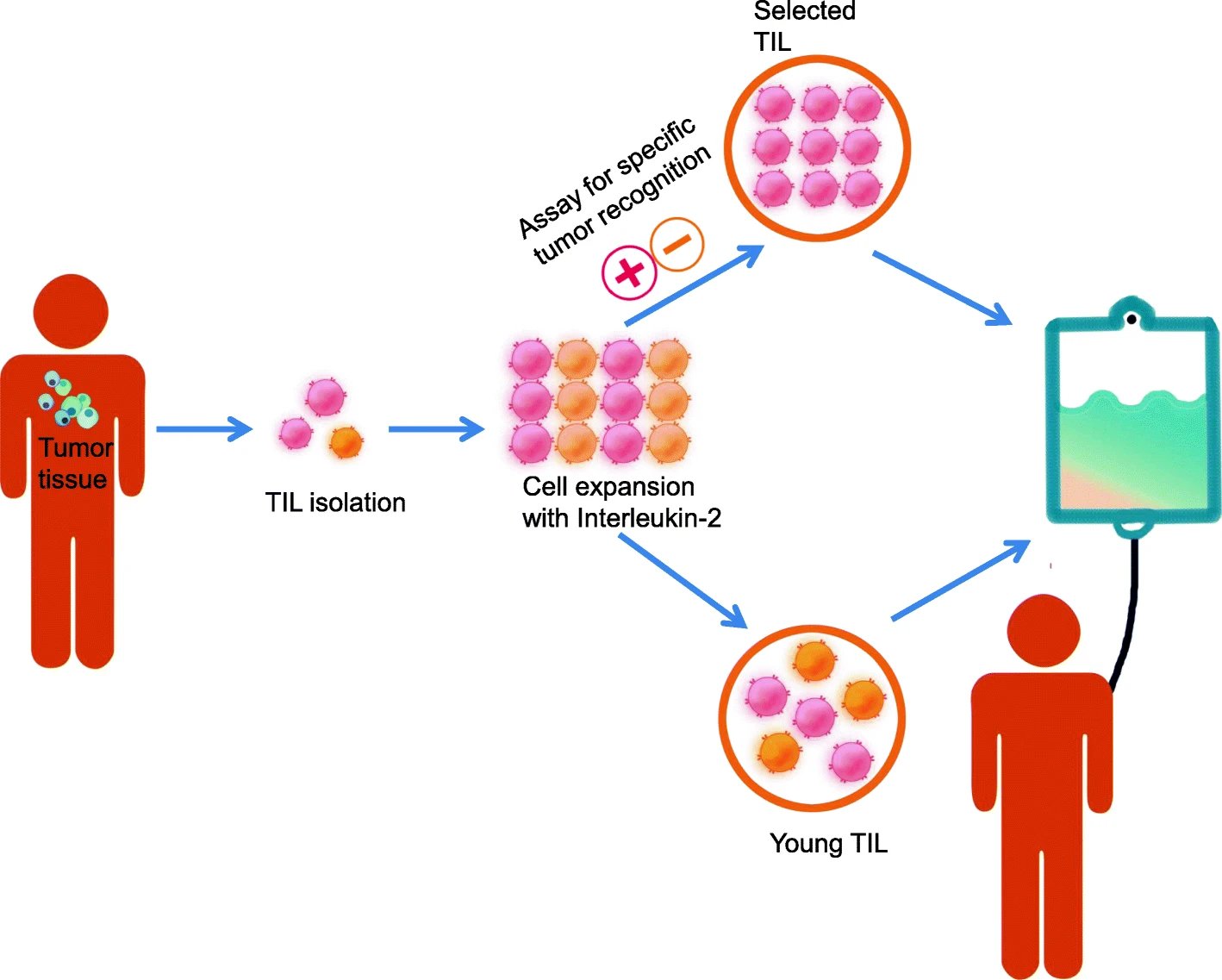
Schematic representation of the production process for TIL therapy. After tumor excision from the patient, tumor is digested into small fragments or a single cell suspension and then expanded in culture with IL-2. In the “selected TIL” approach, expanded cells are selected by their recognition of autologous tumor cells; on the contrary, the “young TIL” approach leaves out this selection step. Then, the TIL culture is expanded to a clinically relevant level and infused back into the patient. From Wang et. al. (2021). Perspectives of tumor-infiltrating lymphocyte treatment in solid tumors. BMC Med 19, 140 (2021); doi.org/10.1186/s12916-021-02006-4. Used under Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Conclusions
For immuno-oncology to maximize its potential, immunotherapies need to be personalized by identifying patient specific immunosuppressive mechanisms and the targeting of specific neoantigens. In addition to the single therapeutic drug strategies discussed above, there are several combined personalized therapies in the early phases of clinical study, with some of them showing highly promising safety and efficacy, such as the combined use of CAR-T cells with a personalized oncolytic vaccine.