Cancer research has evolved significantly over the years, driven by the need for more precise and efficient methods to study tumor biology, identify biomarkers, and develop targeted therapies. One of the most impactful innovations in this field is the Tumor Tissue Microarray (TMA), a high-throughput platform that enables the simultaneous analysis of multiple tumor samples within a single experiment. TMAs have become an indispensable tool for researchers, offering a systematic, scalable, and cost-effective way to study cancer pathology, validate drug targets, and accelerate therapeutic advancements.
Traditional tissue analysis methods involve examining individual tissue samples through histopathology, immunohistochemistry (IHC), and molecular assays. However, these conventional approaches are labor-intensive, expensive, and often limited by sample availability. TMAs address these limitations by allowing researchers to analyze hundreds of tissue specimens in parallel, ensuring greater efficiency, reproducibility, and statistical robustness in experimental studies. By consolidating multiple tissue samples into a single paraffin block, TMAs reduce reagent consumption and optimize laboratory workflows, making large-scale studies feasible.
A key advantage of TMAs is their role in biomarker research—the identification and validation of molecular indicators that help in cancer diagnosis, prognosis, and treatment selection. The ability to examine biomarker expression across a diverse set of tumor tissues enhances the reliability of research findings and facilitates the translation of discoveries into clinical applications. Moreover, TMAs serve as a bridge between preclinical research and drug development, allowing pharmaceutical companies to screen potential drug targets efficiently and evaluate the effectiveness of emerging therapies.
With the increasing emphasis on precision medicine, where treatments are tailored to individual patients based on their molecular and genetic profiles, TMAs have become an essential component of oncology research. By enabling researchers to correlate molecular data with clinical outcomes, TMAs contribute to the development of targeted therapies that improve patient response rates and reduce unnecessary treatments.
In this article, we delve into the fundamentals of Tumor Tissue Microarrays, exploring their construction, applications, and importance in cancer research. We discuss how TMAs aid in biomarker discovery, enhance drug development, and contribute to personalized medicine. Additionally, we examine how researchers can apply the data obtained from TMAs to drive the next steps in clinical and translational research, ultimately improving cancer diagnosis, treatment, and patient care.
Understanding Tumor Tissue Microarrays (TMAs)
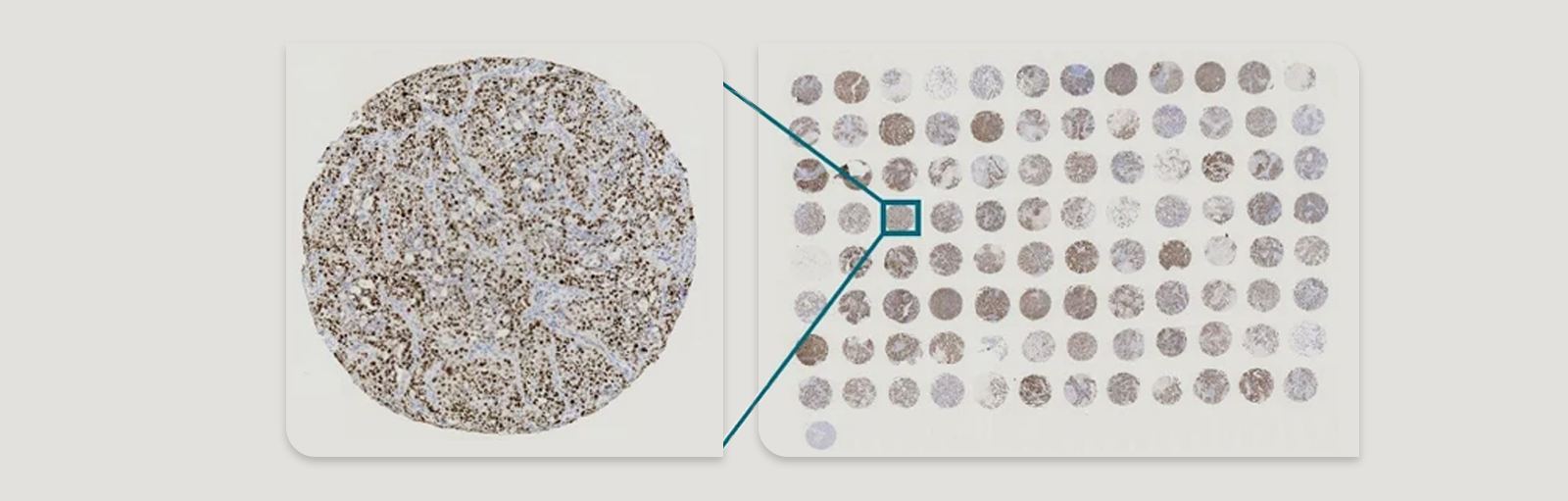
A Tumor Tissue Microarray (TMA) is a powerful research tool designed to enhance the efficiency of histopathological and molecular analyses in oncology. TMAs consist of small tissue core samples, typically extracted from tumor specimens of different patients or experimental models, and embedded into a single paraffin block in a grid-like arrangement. This innovative technique allows for the simultaneous analysis of multiple tissue specimens under uniform experimental conditions, facilitating large-scale studies on cancer biomarkers, drug targets, and therapeutic responses.
How Are TMAs Constructed?
The construction of a TMA follows a precise and systematic process:
Selection of Donor Tissue Blocks
- Researchers first identify representative tumor samples from a tissue biobank or pathology archive. These donor blocks may include normal tissue, primary tumors, and metastatic lesions to allow for comprehensive comparative studies.
Tissue Core Extraction
- A tissue microarrayer (a specialized instrument) extracts small cylindrical cores (typically 0.6–2 mm in diameter) from the chosen donor blocks.
- Each core is carefully selected to ensure it represents key histopathological features of the tumor, such as tumor grade, cellular composition, and biomarker expression.
Arrangement into a Recipient Block
- The extracted tissue cores are systematically arranged into a recipient paraffin block in a pre-defined pattern, allowing each core to be uniquely identified based on its location.
- This structured format ensures that each sample can be tracked and correlated with clinical and pathological data.
Sectioning and Slide Preparation
- Thin sections (4–5 µm) are cut from the TMA block using a microtome and mounted onto glass slides for downstream analysis.
- Multiple sections can be prepared from a single TMA, allowing for repeated testing and cross-validation across different experimental conditions.
Staining and Analysis
- TMAs undergo various analytical techniques, including:
- Immunohistochemistry (IHC) to detect protein expression.
- Fluorescence in situ hybridization (FISH) for gene amplification studies.
- RNA in situ hybridization (RNA-ISH) for transcriptomic analysis.
- Next-generation sequencing (NGS) or polymerase chain reaction (PCR) for genetic profiling.
Key Features of TMAs
- High-Throughput Analysis: TMAs allow the study of hundreds of samples simultaneously, making them ideal for large-scale biomarker validation and cancer studies.
- Standardization and Reproducibility: Since all samples within a TMA undergo identical experimental conditions, variability is significantly reduced compared to individual tissue slide analysis.
- Cost and Time Efficiency: By consolidating multiple samples onto a single slide, TMAs minimize reagent usage, reduce processing time, and lower overall research costs.
- Enhanced Statistical Power: The inclusion of numerous tumor samples enables researchers to obtain statistically significant insights into biomarker prevalence and drug response across diverse patient populations.
Comparing TMAs to Traditional Tissue Analysis
| Feature |
Traditional Tissue Analysis |
Tumor Tissue Microarrays (TMAs) |
| Number of Samples |
One tissue per slide |
Hundreds of tissues per slide |
| Reagent Consumption |
High |
Significantly reduced |
| Time Required for Analysis |
Time-consuming |
High-throughput, faster results |
| Experimental Variability |
Higher due to sample-to-sample differences |
Lower, as all samples are treated identically |
| Cost |
Expensive due to individual processing |
More cost-effective |
Why Are TMAs Important?
Tumor Tissue Microarrays have transformed cancer research by enabling efficient, large-scale biomarker discovery and drug development. Their ability to integrate diverse tumor samples into a single, manageable platform has made them an essential tool for oncology research, clinical diagnostics, and precision medicine. By allowing direct comparison of tumor characteristics under controlled conditions, TMAs provide valuable insights into tumor biology, disease progression, and therapeutic responses, ultimately guiding the development of more effective cancer treatments.
How Do TMAs Work?
The Tumor Tissue Microarray (TMA) process is a highly structured and efficient method that enables researchers to analyze multiple tumor specimens simultaneously. By condensing a large number of tissue samples into a single paraffin block, TMAs streamline histological and molecular analyses, significantly reducing both cost and labor compared to traditional tissue slide preparation.
The TMA workflow involves several critical steps, ensuring that tissue samples are processed in a reproducible and high-throughput manner.
Sample Selection: Choosing the Right Tissue Specimens
The first step in TMA construction involves the careful selection of tumor specimens that will be included in the array. Researchers identify donor paraffin-embedded tissue blocks from biobanks, pathology archives, or clinical studies. The selection process ensures that:
- The chosen specimens are representative of the tumor type and exhibit key histopathological characteristics.
- Multiple tumor types or subtypes can be included for comparative analysis.
- Sufficient clinical and pathological data (e.g., patient demographics, disease stage, treatment history) is available for correlation.
To maximize research outcomes, TMAs may incorporate normal tissue controls, tumor-adjacent tissues, and metastatic lesions to enable comprehensive comparisons.
Tissue Core Extraction: Isolating Small Tissue Sections
Once donor blocks are selected, the next step is tissue core extraction, where small cylindrical cores (0.6–2 mm in diameter) are removed from specific regions of interest within the tissue samples. This is performed using a specialized instrument known as a tissue microarrayer, which precisely extracts tissue cores while maintaining structural integrity.
- The size of the core (typically 0.6 mm–2 mm) depends on the research objective and available tissue material.
- Multiple cores from the same tumor can be extracted to account for tumor heterogeneity.
- Core selection is guided by expert pathologists to ensure that the extracted regions contain viable tumor tissue and avoid necrotic or damaged areas.
This meticulous extraction process ensures that each sample in the TMA provides high-quality and reproducible data for downstream analyses.
TMA Block Construction: Arranging the Tissue Cores
After extraction, the tissue cores are systematically arranged into a recipient paraffin block in a structured grid pattern. This organization is pre-planned to maintain sample traceability and allow for accurate comparison of different tumor types.
- The recipient paraffin block acts as a housing matrix, embedding multiple cores in a precise layout.
- Each tissue sample is assigned a unique identifier to correlate its position in the array with associated clinical data.
- TMAs can include hundreds of tissue cores, making them ideal for large-scale studies.
By consolidating multiple tissue samples into a single block, TMAs enable standardized processing and analysis, reducing variability and improving data comparability.
Sectioning and Slide Preparation: Creating TMA Slides for Analysis
Once the TMA block is assembled, thin sections are cut using a microtome (a precision-cutting instrument). These 4–5 µm sections are then mounted onto glass slides for high-throughput staining and molecular analysis.
- Multiple sections can be prepared from a single TMA block, allowing researchers to perform various assays on identical samples.
- Sections are preserved in optimal conditions to ensure tissue integrity and molecular stability.
High-Throughput Analysis of TMAs
TMA slides are subjected to various analytical techniques to examine molecular and histopathological features, including:
Immunohistochemistry (IHC) – Protein Expression Analysis
- Used to detect and quantify specific proteins within tumor samples.
- Involves staining tissue sections with antibodies that bind to target proteins.
- Commonly used to assess oncogenic biomarkers (e.g., HER2, PD-L1, Ki-67) in cancer research.
Fluorescence In Situ Hybridization (FISH) – Genetic Alteration Detection
- Uses fluorescently labeled DNA probes to detect gene amplifications, deletions, or rearrangements.
- Important for identifying genetic mutations linked to targeted cancer therapies.
RNA In Situ Hybridization (RNA-ISH) – Transcriptomic Analysis
- Detects mRNA expression levels within tissue samples.
- Helps study gene regulation and tumor-specific transcriptomic signatures.
Next-Generation Sequencing (NGS) and Polymerase Chain Reaction (PCR)
- Used for in-depth genomic profiling of tumors.
- Enables identification of driver mutations, epigenetic modifications, and mutational burden in cancer.
Advantages of TMA-Based Analysis
The integration of TMAs into cancer research provides several advantages over traditional histopathology methods:
| Feature |
Traditional Tissue Analysis |
Tumor Tissue Microarrays (TMAs) |
| Number of Samples per Slide |
One tissue per slide |
Hundreds of tissues per slide |
| Reagent Consumption |
High |
Reduced |
| Time Required for Analysis |
Labor-intensive |
High-throughput and efficient |
| Experimental Variability |
Higher due to sample-to-sample differences |
Lower, as all samples are processed under
identical conditions |
| Cost |
Expensive due to individual sample processing |
|
The TMA technique has transformed cancer research by enabling high-throughput, large-scale tissue analysis with minimal cost and experimental variability. By consolidating multiple tumor samples into a single platform, TMAs streamline biomarker discovery, drug development, and precision oncology research.
This efficient methodology allows researchers to analyze hundreds of tissue specimens simultaneously, making it an indispensable tool in modern oncology. Through continued advancements in digital pathology, artificial intelligence, and multiplexed molecular assays, TMAs will remain a cornerstone of cancer research and drug discovery, shaping the future of personalized medicine.
Advancing Biomarker Discovery
Biomarkers are crucial for diagnosing cancer, predicting disease progression, and selecting appropriate therapies. TMAs help in:
- High-throughput biomarker screening: TMAs allow researchers to examine multiple biomarkers simultaneously across numerous patient samples.
- Correlation with clinical data: TMAs integrate molecular findings with patient demographics, treatment history, and clinical outcomes, enhancing the reliability of biomarker validation.
Enhancing Drug Development and Personalized Medicine
TMAs support drug development by:
- Identifying therapeutic targets: Analyzing protein and gene expression levels to pinpoint druggable targets.
- Assessing treatment response: Evaluating how different tumors react to specific drugs.
- Stratifying patients for clinical trials: Ensuring that trial participants have the required molecular profile for targeted therapies.
Improving Research Efficiency and Cost-Effectiveness
Compared to conventional histopathology techniques, TMAs provide:
- Standardization of experimental conditions: All tissue samples undergo identical testing conditions, reducing variability.
- Cost savings: By consolidating samples onto a single slide, TMAs reduce reagent consumption and processing time.
- Increased statistical power: The large number of samples per experiment improves the robustness of research findings.
Applying TMA Data for the Next Steps in Research and Treatment
Translating TMA Insights into Clinical Applications
TMA data is instrumental in:
- Developing diagnostic tests: Biomarker expression patterns identified through TMAs can lead to improved diagnostic assays.
- Predicting therapy outcomes: Helps determine which patients are most likely to benefit from targeted therapies.
Using TMAs for Target Validation
By correlating molecular findings with patient responses, TMAs enable:
- Identification of promising drug targets: Helps prioritize candidate targets for further drug development.
- Validation of preclinical drug testing results: Ensures that experimental findings translate into clinically meaningful insights.
Role in Personalized and Precision Medicine
Personalized medicine tailors treatments based on individual patient characteristics. TMAs contribute to this field by:
- Stratifying patients based on biomarker expression: Ensuring that only patients with responsive tumors receive specific therapies.
- Facilitating development of combination therapies: Identifying which drugs work best together based on tumor molecular profiles.
Tumor Tissue Microarrays in Drug Discovery
Screening for Novel Drug Targets
TMAs facilitate drug discovery by:
- Profiling tumor heterogeneity: Identifying variations in tumor biology that may impact drug efficacy.
- Validating potential therapeutic targets: Assessing the relevance of specific proteins or genes in cancer progression.
Evaluating Drug Efficacy and Resistance
TMAs help researchers understand:
- Which tumors respond to specific treatments: Enabling better drug selection for individual patients.
- Mechanisms of drug resistance: Identifying molecular changes that cause treatment failure.
Applications in Immunotherapy and Combination Therapies
- TMA-based studies in immuno-oncology: Evaluating biomarkers such as PD-L1 to predict response to immune checkpoint inhibitors.
- Combination therapy strategies: TMAs help determine which drugs work best in synergy by assessing biomarker co-expression patterns.
Challenges and Future Perspectives
Tumor Tissue Microarrays (TMAs) have significantly improved cancer research and drug discovery, but like any scientific tool, they come with inherent challenges. Researchers are continuously working on refining TMA methodologies to overcome these limitations and enhance their applicability. Furthermore, advancements in technology, including artificial intelligence, digital pathology, and multiplexed imaging, are opening new avenues for TMA applications. In this section, we explore the key challenges of TMAs, how emerging technologies are addressing these issues, and the future potential of TMAs in oncology and drug discovery.
Limitations of TMAs in Research
While TMAs offer substantial advantages, there are several limitations that researchers must consider when designing studies and interpreting results.
Heterogeneity of Tumor Samples
- One of the most significant challenges of TMAs is the intratumoral heterogeneity present in many cancers.
- Tumors are highly heterogeneous, meaning different areas of the same tumor may exhibit varied biomarker expression, genetic mutations, or drug response profiles.
- Since TMAs rely on small tissue cores (0.6–2 mm in diameter) extracted from a tumor, there is a risk that the selected core may not accurately represent the full biological complexity of the tumor.
- Solution: To mitigate this limitation, researchers often extract multiple cores from different tumor regions to capture heterogeneity more accurately.
Sample Selection Bias
- The selection of donor tissue samples can introduce bias, affecting the reproducibility and generalizability of research findings.
- Different tissue fixation, processing, and storage conditions across institutions may result in inconsistencies in TMA sample quality.
- Variability in tissue preservation techniques (e.g., formalin-fixed paraffin-embedded vs. frozen samples) can impact biomarker integrity and research outcomes.
- Solution: Implementing standardized protocols for tissue selection, fixation, and processing can improve reproducibility across different research groups.
Data Interpretation Complexity
- The vast amount of data generated from TMAs, especially in multiplexed and high-throughput studies, requires advanced computational tools for analysis.
- Biomarker quantification and expression pattern recognition require image analysis software and expertise in bioinformatics.
- High-dimensional datasets from TMAs demand statistical modeling and machine learning approaches for accurate interpretation.
- Solution: The integration of AI-driven image analysis and big data analytics is helping researchers extract meaningful insights from complex TMA datasets.
The Future of TMAs in Oncology and Drug Discovery
As TMAs continue to evolve, their applications are expected to expand across various domains, including rare cancer research, precision medicine, and regulatory integration into drug approval pipelines.
Expanding Applications in Rare Cancers
- TMAs offer a unique advantage in studying rare and understudied cancers, where sample availability is limited.
- Researchers can pool tumor samples from different sources to create TMAs that facilitate comparative analysis across multiple rare cancer types.
- Example: TMAs have been instrumental in profiling pediatric cancers, where obtaining large sample cohorts for traditional analysis is challenging.
Integration into Precision Oncology and Personalized Medicine
- TMAs are increasingly being used to stratify patients for targeted therapies by identifying tumors with specific molecular signatures.
- As part of clinical trials, TMAs help predict which patients will benefit most from novel cancer treatments.
- Example: TMAs have been used to identify HER2-positive breast cancer patients for targeted HER2 inhibitor therapies.
Regulatory Adoption and Standardization
- As TMAs become the gold standard for biomarker validation, regulatory agencies may incorporate them into drug approval processes.
- The FDA and EMA are evaluating the role of TMAs in companion diagnostics, ensuring that biomarkers identified through TMAs can guide clinical decision-making.
- Example: TMAs have been used in companion diagnostics for PD-L1 inhibitors (immune checkpoint blockade therapy) in lung cancer and melanoma.
Despite certain limitations, Tumor Tissue Microarrays (TMAs) have transformed oncology research, enabling high-throughput biomarker discovery, efficient drug target validation, and precision medicine applications. Emerging technologies such as AI-driven image analysis, digital pathology, and multiplexed imaging are further enhancing the capabilities of TMAs, addressing challenges related to tumor heterogeneity, sample bias, and data interpretation.
As TMAs continue to evolve, they are expected to play a more significant role in rare cancer research, personalized treatment strategies, and regulatory drug development pipelines. By integrating TMAs with next-generation sequencing, artificial intelligence, and machine learning, the future of cancer research and precision oncology will be increasingly data-driven and patient-specific.
Through continued advancements, TMAs will remain a cornerstone technology in oncology, guiding the next generation of cancer diagnostics and therapeutics to improve patient outcomes worldwide.
Conclusion
Tumor Tissue Microarrays (TMAs) are transforming cancer research by enabling high-throughput, cost-effective analysis of biomarkers, drug targets, and treatment responses. Their role in biomarker discovery, drug development, and personalized medicine underscores their significance in advancing oncology.
By allowing the simultaneous examination of hundreds of tumor samples under identical experimental conditions, TMAs have significantly improved the efficiency, reproducibility, and scalability of oncology studies.
One of the most impactful contributions of TMAs is in biomarker discovery, where they facilitate the identification of molecular markers that can guide cancer diagnosis, prognosis, and targeted therapy development. By integrating TMAs into large-scale research efforts, scientists and clinicians can correlate biomarker expression with clinical outcomes, paving the way for precision medicine and personalized treatment strategies.
In drug development, TMAs play a crucial role in validating novel therapeutic targets, assessing drug efficacy, and understanding mechanisms of resistance. Pharmaceutical companies and research institutions leverage TMAs to accelerate the screening and validation of cancer drugs, ultimately shortening the timeline from discovery to clinical application. Their ability to optimize patient stratification for clinical trials ensures that new therapies reach the right patient populations, maximizing treatment benefits and minimizing unnecessary exposure to ineffective drugs.
Ultimately, TMAs are not just revolutionizing laboratory-based research—they are bridging the gap between scientific discovery and clinical application, improving drug development pipelines, and enhancing patient care outcomes. As technology advances, the future of TMAs in cancer research will be defined by their ability to drive more precise, efficient, and impactful discoveries, leading to better-targeted therapies and improved survival rates for cancer patients worldwide.