Revolutionizing Cancer Drug Combination Discovery with AI: Introducing SynAI

I am excited to share with you a significant advancement in the field of cancer research, the culmination of recent efforts by my team and me. We have published our study on "SynAI" in the journal "Bioinformatics Advances" (November 2023). SynAI is an AI-driven platform designed to predict the synergistic effects of cancer drug combinations, poised to fundamentally change how we discover and evaluate cancer treatments.
Understanding SynAI: A Major Leap in Cancer Treatment
At its heart, SynAI is about predicting the combined effects of cancer drugs, known as drug synergism. This AI-driven solution is flexible and innovative, focusing on early-stage drug discovery to identify the therapeutic potential of various compound combinations.
Unlike traditional approaches, SynAI does not rely on actual drug combinations or specific cell lines. Instead, it utilizes the Simplified Molecular Input Line Entry System (SMILES) sequences of compounds. Our AI model, trained with over 1.2 million in vitro tests across different cancer cell lines, predicts potential synergisms or antagonisms between drug combinations without the need for costly and time-consuming physical synthesis or structural analysis.
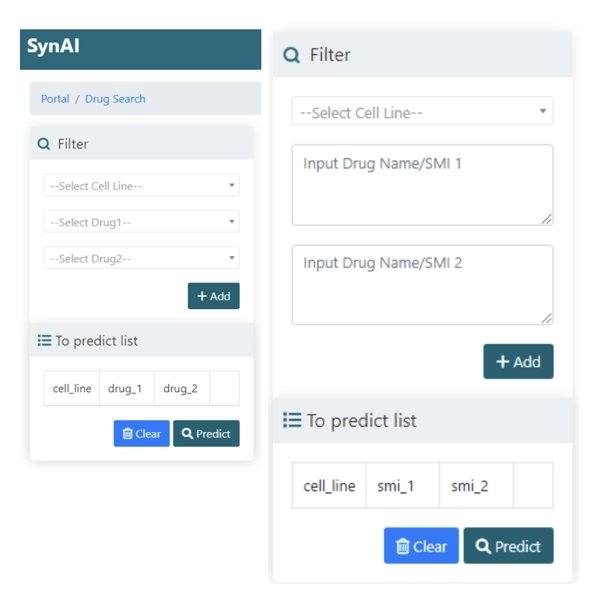
Figure 1. Samples of the SynAI interface, where you can select the target cell
line and the drug combination of interest for drug synergy prediction.
How SynAI Benefits Cancer Research
- Cost-efficiency: By using only SMILES sequences, SynAI significantly reduces the costs associated with candidate screening during drug development.
- Time-saving: The computational predictions eliminate the need for physical compound synthesis, speeding up the initial screening process.
- Flexibility and Adaptability: Our AI model is retrainable, meaning it can continuously adapt and improve with new data, making it a future-proof tool in the battle against cancer.
SynAI's Unique Features
- Multi-input Modes: SynAI offers four input modes to cater to different research needs and allows for bulk processing through an API.
- Robust AI Core: The platform’s AI core, built on a multi-layer perceptron network, has undergone rigorous training and testing.
- Predictive Accuracy: Our model uniquely handles SMILES sequences, ensuring accurate predictions regardless of the compound order.
- Retrainable and Adaptive AI Core: SynAI's AI core is not static; it's retrainable and adaptive. This means that any new experimental data can be seamlessly integrated into the core to reinforce prediction coverage and enhance accuracy over time. This dynamic feature ensures that SynAI continuously improves its performance as more data becomes available, making it a powerful tool for cutting-edge research.
The Future of Personalized Medicine
The potential of SynAI extends beyond its current applications. We plan to incorporate organoid and clinical data, paving the way for personalized medicine. Imagine a future where cancer treatment is tailored to the individual, maximizing therapeutic efficiency and minimizing side effects.
Conclusion: A Step Towards a Brighter Future
In conclusion, SynAI stands out not only for its current capabilities but also for its potential to evolve with ongoing research. It's more than a platform; it's a beacon of hope in our continuous fight against cancer.
Cite this Article
Yan, K., (2024) Revolutionizing Cancer Drug Combination Discovery with AI: Introducing SynAI - Crown Bioscience. https://blog.crownbio.com/revolutionizing-cancer-drug-combination-discovery-with-ai