 Genetically engineered mouse models (GEMM) were reviewed in a recent post, as immunocompetent, murine immunity models for immuno-oncology assays. While highly useful for monitoring cancer progression and mechanism of action studies, the spontaneous nature of GEMM tumors makes them complex and costly models for efficacy studies.
Genetically engineered mouse models (GEMM) were reviewed in a recent post, as immunocompetent, murine immunity models for immuno-oncology assays. While highly useful for monitoring cancer progression and mechanism of action studies, the spontaneous nature of GEMM tumors makes them complex and costly models for efficacy studies.
This post reviews the steps that have been made to overcome these limitations – utilizing GEMM derived resources, and also next generation models.
GEMM Limitations: Complex and Costly Efficacy Study Platform
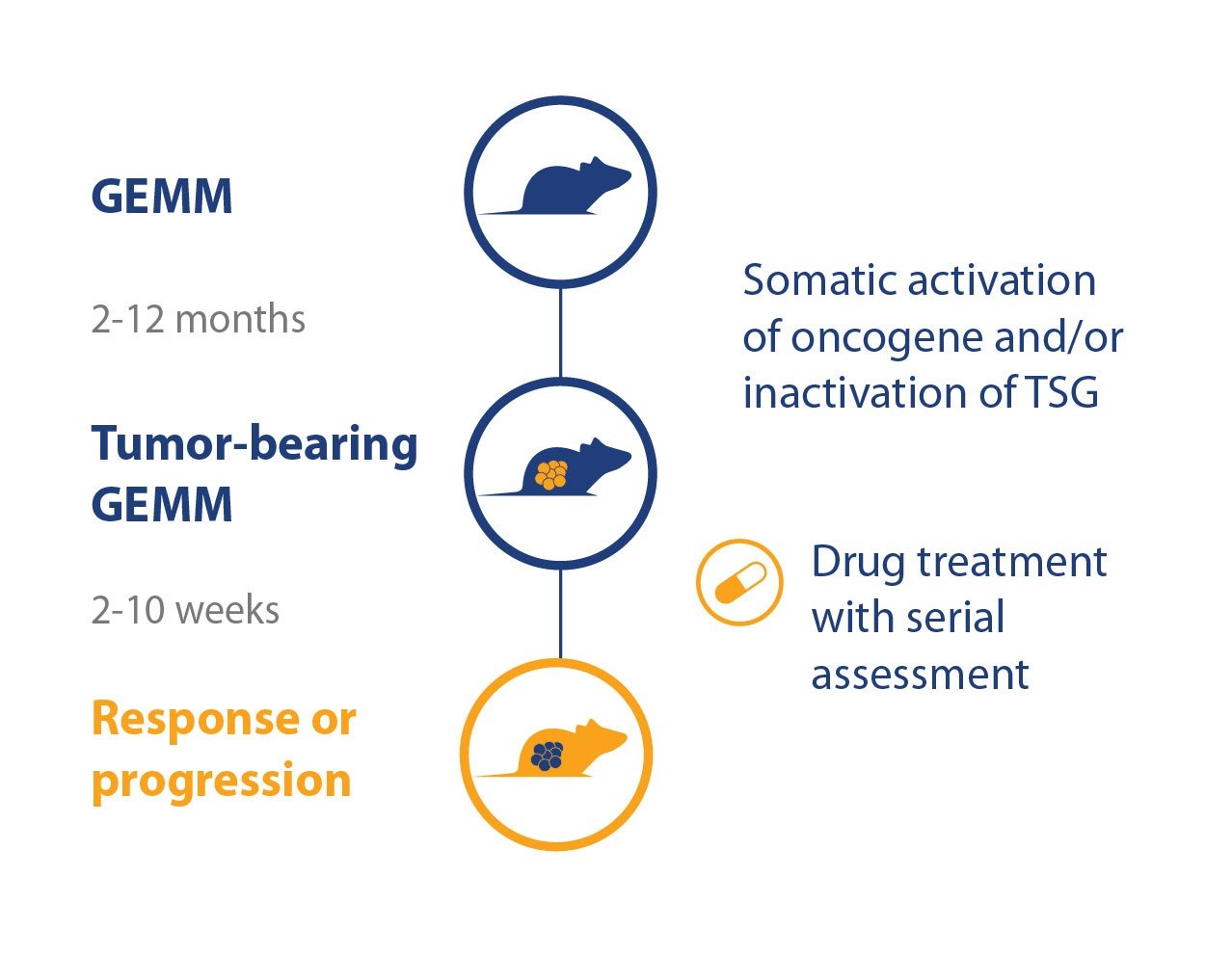
With the wide variety of well-characterized GEMM available, featuring tumors with a clear molecular pathogenesis of disease within an immunocompetent setting, it’s no surprise many people would like to use them for immunotherapy efficacy assessment. However, with long latency periods, mice developing disease at different stages, and 100% penetrance not achievable, this rapidly becomes complex and costly.
Mouse Allograft Models: A Murine Version of PDX, “Primary” in Nature
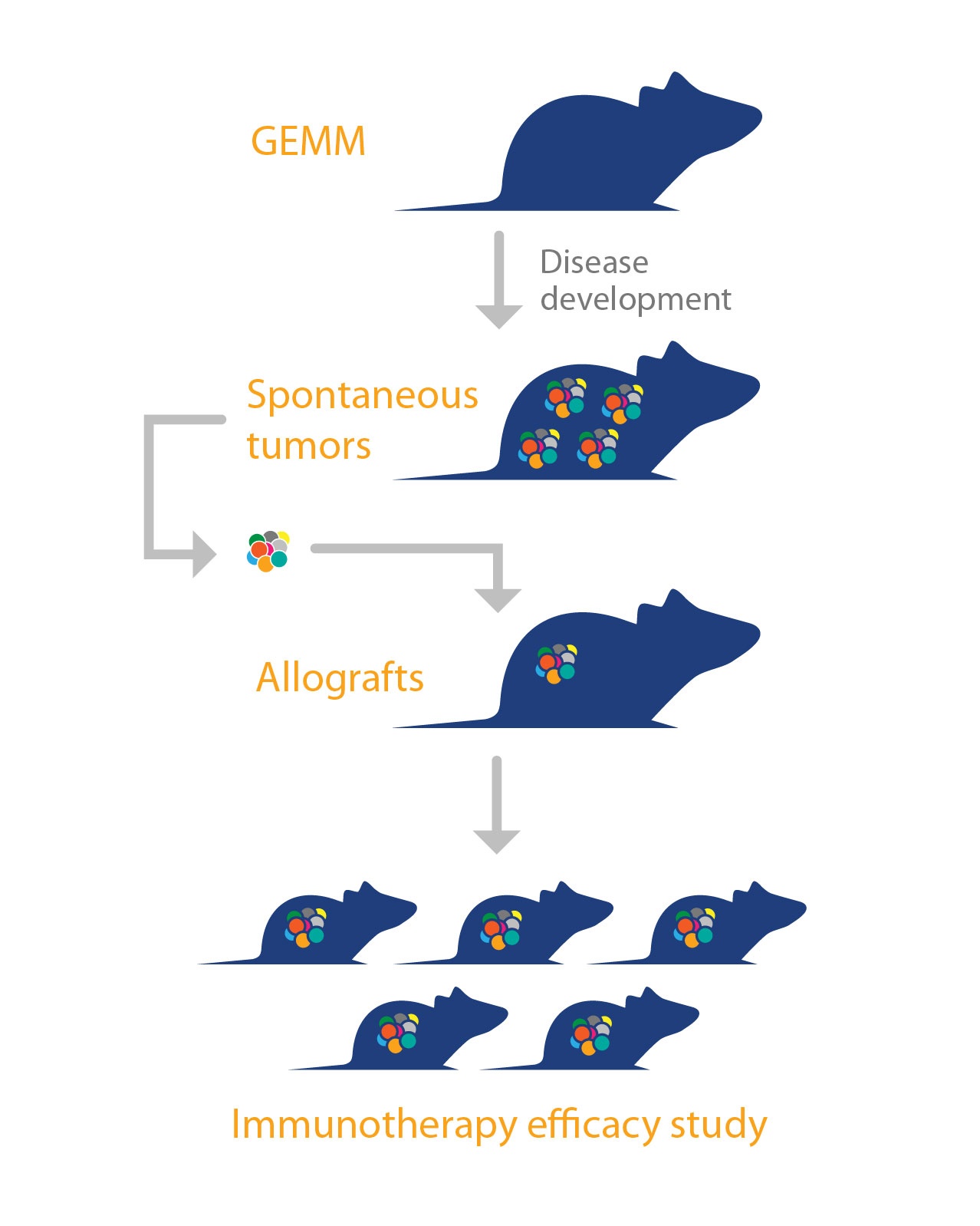
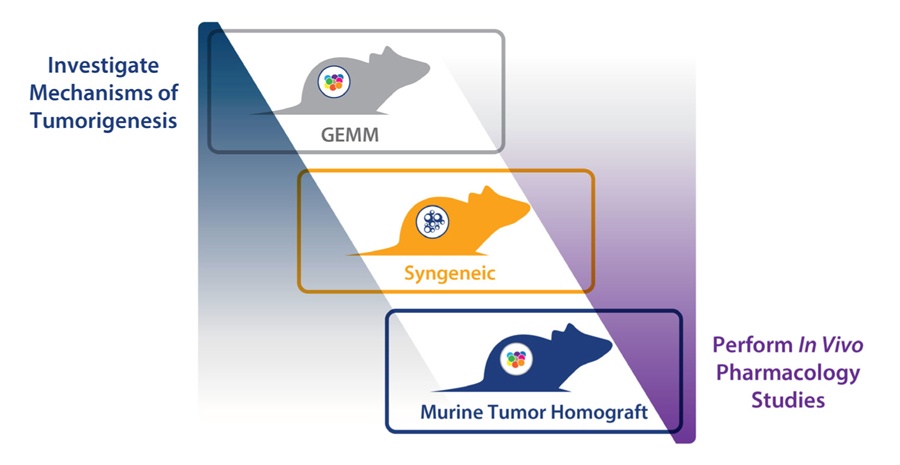
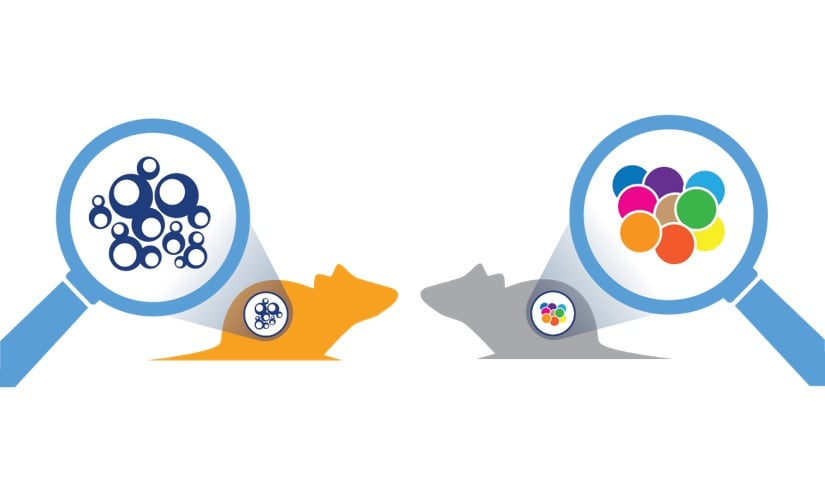
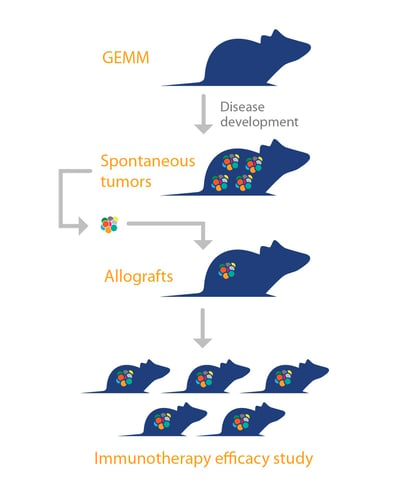
To overcome this, a “murine version of patient-derived xenografts (PDX)” has been developed, using GEMM tumors. The platform is comprised of allografts of spontaneous tumors from GEMM, engrafted in syngeneic mice with complete immunocompetency. Like PDX, these murine tumors are “primary” in nature, they are not manipulated or adapted to grow in vitro, and therefore mirror the original tumor histopathology and genetic profile. This means they can recapitulate the original disease better than models such as syngeneics, where immortalized cell lines (which can drift from original disease) are used to create tumors.
Combining GEMM Strengths with Operational Simplicity
These allograft models, combine the strengths of GEMM (such as various differentiation phenotypes, clinically relevant disease pathways with oncogenic drivers, rich microenvironments, and cancer stem cell driven disease) with an improved operational simplicity, consistency, and the robust growth needed for pharmacology research and efficacy studies.
As large collections of GEMM exist, it means that (similar to PDX), a large library of mouse allograft models across a range of cancer types and subtypes could also be developed for immunotherapy studies. This would allow population studies similar to PDX mouse clinical trials, and should provide highly predictive preclinical efficacy models, also enabling discovery of predictive biomarkers.
Next Generation Models: More Sophisticated with Shortened Timelines
Another way to overcome GEMM limitations is through next generation models:
- Conditional GEMM: more sophisticated compared with traditional GEMM, these models allow induction of tumor onset and progression in a cell lineage/tissue specific manner
- More recent advances in technology (e.g. CRISPR/Cas 9 gene editing, RNAi, viral delivery) have resulted in GEMM with on and off gene manipulation, which can sequentially or simultaneously disrupt multiple oncogenes and/or tumor suppressor genes to truly mimic human disease development
Overall, this has shortened timelines compared with tumor development in traditional GEMM.
GEMM remain a useful tool for immunotherapy research, hopefully this post has shown how their limitations can be overcome to develop improved models to enhance immunotherapy assessment.
Further reading on where murine allograft models fit into an immuno-oncology model portfolio can be found here:
Li et al. Experimental animal modeling for immuno-oncology. Pharmacol Ther 2017;173: 34-46.