 Immunocompetent models are necessary for the preclinical assessment of novel immunotherapies, but multiple murine model platforms are available for the evaluation of cross reactive and surrogate agents. Here we look at syngeneics, GEMM, and murine tumor homograft models and how to choose the best model for your immuno-oncology study.
Immunocompetent models are necessary for the preclinical assessment of novel immunotherapies, but multiple murine model platforms are available for the evaluation of cross reactive and surrogate agents. Here we look at syngeneics, GEMM, and murine tumor homograft models and how to choose the best model for your immuno-oncology study.
Immunocompetent Murine Models for Preclinical Research
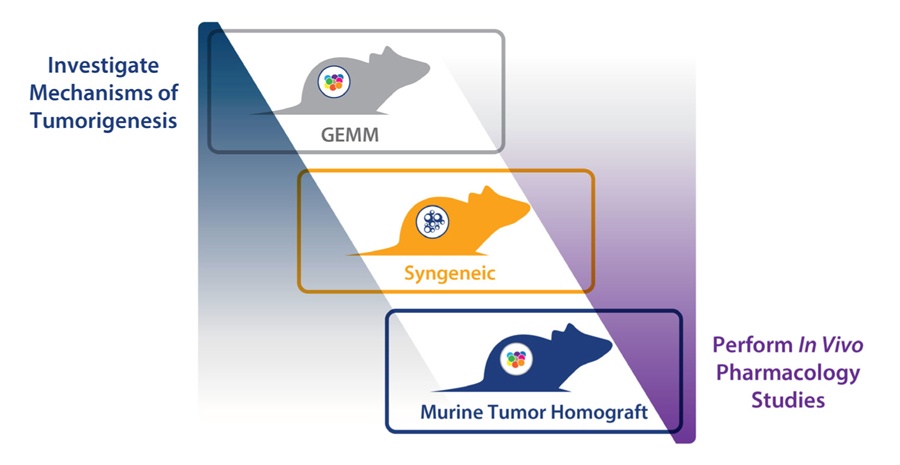
There are three main types of immunocompetent murine model available for preclinical drug development. Each model type has their own strengths and limitations, which determines which model is best utilized for different preclinical applications.
The three model types are:
- Syngeneic tumor models, allografts of immortalized mouse cancer cell lines which are utilized with mice from the same inbred strain to prevent tumor rejection
- Genetically engineered mouse models (GEMM), with transgenic, knock in, or knockout models developed around specific oncogenes or tumor suppressors. This results in mouse models with spontaneously arising tumors, mimicking the genetic events observed in human disease
- Murine tumor homograft models, syngeneic homografts of spontaneous mouse tumors derived from GEMMs or carcinogen-induced tumors.
Choosing the correct model for any given study is based on a combination of factors, with study design and outcome at the top of the list.
Model Choice Based on Study Design and Outcomes
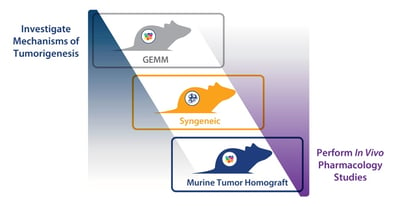
Modelling Tumorigenesis Mechanisms with GEMM
The first point to consider when choosing an immunocompetent murine model is the outcome of the study – efficacy or understanding mechanisms of tumorigenesis.
When investigating tumorigenesis or validating the function of a specific target, GEMMs are the ideal choice. Tumors develop due to specifically engineered mutations, meaning that there is a clear molecular pathogenesis of disease around a known mutated genetic feature. Available models have also been highly characterized through many years of study, with historical comparison data available. Other relevant features include tumor stroma and a mouse tumor replicating the location of the patient tumor.
When moving to efficacy studies, GEMM are no longer appropriate, mainly due to a whole raft of issues around spontaneous tumor development (e.g. tumors aren’t synchronized, long latency periods, rolling enrollment, etc.). This leaves two potential options – syngeneics or murine tumor homografts.
The final choice is determined by a number of other study design factors. Both models can be established subcutaneously or as orthotopic models to facilitate easy disease tracking or more patient-comparable disease, respectively.
Syngeneics and Murine Tumor Homografts for Efficacy Studies
Syngeneics in Immunotherapy Assessment
Syngeneics have been the workhorse of immunotherapy assessment. They provide a well understood, robust and reproducible platform, and a lot of historical and benchmark data. Tumor growth can be synchronized for efficient efficacy studies. Syngeneics are highly useful for combination immunotherapy assessment, and high throughput testing. Downsides include potential genetic drift from original disease, and limited numbers of models meaning all cancer types might not be available for study.
Murine Tumor Homografts for Specific Targeting
Murine tumor homografts are a newer platform, designed to combine the features of GEMM with the operational simplicity of syngeneics for more patient relevant efficacy studies or studies around a specific genetic feature. These models harvest GEMM spontaneous tumors and engraft them in mice of the same strain, followed by expansion and archive. This creates a murine platform similar to patient-derived xenografts (PDX), where cohorts of models are engrafted in a highly reproducible and robust manner to allow larger scale evaluation of immunotherapies, and with models which conserve original murine tumor histo- and molecular pathology.
One downside compared with syngeneics is that these models are more complex, which can complicate analysis of response data. This means that syngeneics are likely to be chosen for simple combination agent efficacy studies.
Murine tumor homografts may be needed when targeting a specific genetic feature, or when a cancer type is not available from a syngeneic collection.
Additional Model Selection Factors
Beyond study outcomes, there are a range of other model features which play an important role in model selection.
Tumor Stroma
Relevance to human disease can be a major factor when choosing a preclinical model, including the presence of a tumor stroma. GEMM spontaneous tumors recapitulate the location of a human tumor, and are therefore surrounded by a tumor microenvironment, with tumor stroma and recruited immune cells.
As tumor homografts are transplanted from GEMM they conserve some stromal elements and involvement. The tumors also have a 3D architecture, complex structure, and heterogeneity which are not found in the homogenous syngeneic tumors derived from cancer cell lines.
Tumor Mutational Load
Tumor homograft models are more similar to human disease than syngeneics when examining tumor mutational load. Murine tumor homograft models have a lower mutational load due to the spontaneous origins of the parental tumor, and fewer passages of the tumor homograft models compared to immortalized cell lines.
The higher mutational load of syngeneics is well-documented. This is part of the reason why a large immune response occurs after syngeneic engraftment and why immune checkpoint inhibitors work so well against syngeneics.
These factors can mean that instead of choosing between syngeneics and murine tumor homografts for efficacy studies, they can be run sequentially. First, test a new agent against syngeneics which are likely to provide a good response. Then move up to a more human disease-relevant tumor homograft, for more rigorous assessment.
Bioluminescent Imaging
If you are looking to assess the response to a novel agent through imaging, syngeneics are a good choice through the establishment of bioluminescent orthotopic variants via stable transduction with luciferase. Bioluminescent imaging (BLI) can bring many advantages to preclinical drug development, including in-life monitoring of tumor burden, non-invasively and at multiple time points. BLI can also help with monitoring tumor take rate, earlier treatment initiation, and in optimizing randomization.
Flexible and Comprehensive Drug Development Programs
With the availability of multiple immunocompetent models, flexible and comprehensive drug development programs can be designed, with complementary preclinical models fitting different needs across research and efficacy studies. GEMM allow valuable studies into mechanisms of tumorigenesis. Syngeneic models are ideal for studies needing high reproducibility and throughput, and murine tumor homografts take a step closer to clinical relevance in efficacy studies.