The field of oncology research demands innovative, multi-dimensional approaches to tackle the complexities of cancer biology, therapeutic responses, and immune interactions. High Content Imaging (HCI) is revolutionizing this space, combining automated microscopy, advanced imaging, and data analytics to provide unparalleled insights into cellular behaviors. Designed for high-throughput applications, HCI accelerates therapeutic compound screening while uncovering mechanisms of resistance and drug responses.
Particularly in immuno-oncology, HCI enables researchers to visualize and quantify intricate immune-cancer cell interactions, advancing the development of immunotherapies that effectively engage the body’s immune system against cancer. This powerful technology is driving breakthroughs in preclinical and translational research, paving the way for more precise and effective cancer treatments.
Fundamentals of High Content Imaging
High Content Imaging combines advanced microscopy with image analysis algorithms to quantify changes in cellular parameters in response to drug treatments or other experimental conditions. Unlike traditional high-throughput screening (HTS), which typically focuses on a single endpoint, HCI captures a multi-dimensional view of cellular states, allowing researchers to analyze parameters like morphology, protein expression, and spatial distribution simultaneously.
Key technological components of HCI include:
Fluorescence Microscopy
Used to label and visualize cellular structures and molecular markers, fluorescence microscopy enables the observation of dynamic processes, such as protein trafficking and signal transduction, at high resolution.
Confocal Imaging
By capturing multiple focal planes, confocal imaging offers better depth resolution and reduces background noise, which is essential for analyzing complex 3D cell models.
Image Software
The software interprets and quantifies data from thousands of images, generating metrics on cell morphology, intensity of specific biomarkers, and interactions between cellular compartments. This quantitative approach supports high-throughput studies while minimizing variability and human error.
Through these technologies, HCI allows for deeper phenotypic analysis, enabling researchers to explore how cancer cells respond to treatment at the molecular and cellular levels. This approach is highly beneficial in oncology, where a single compound can have multi-faceted effects on cell populations and tissues.
Applications of High Content Imaging in Oncology
HCI has transformed oncology research by offering insights into drug mechanisms, resistance profiles, and the complex biology of tumor cells.
Screening for Anti-Cancer Compounds
HCI enables the evaluation of anti-cancer compounds by examining phenotypic changes in cancer cells. Using multi-parametric analysis, researchers can assess parameters like cell proliferation, apoptosis, and DNA damage. By monitoring these factors, HCI helps identify compounds that effectively inhibit cancer cell growth or induce cell death.
Case Study: Recent studies have used HCI to screen libraries of FDA-approved drugs for off-target effects in cancer treatment. By quantifying changes in cell shape, motility, and biomarker expression, these studies have identified promising drug repurposing opportunities in oncology.
Drug Resistance Studies
Drug resistance is a major hurdle in cancer treatment. HCI enables researchers to visualize and analyze how cancer cells adapt to therapeutic agents over time, thereby identifying the cellular and molecular mechanisms underlying resistance.
Example: HCI has been used to investigate the role of epithelial-mesenchymal transition (EMT) in resistance to targeted therapies. By observing changes in cell morphology and migration, researchers have gained insights into how EMT contributes to resistance, paving the way for combination therapies that prevent or overcome these adaptations.
Tumor Heterogeneity Analysis
Tumors are highly heterogeneous, with subpopulations of cells displaying different characteristics and treatment responses. HCI provides a way to profile individual cells within a population, allowing for the detection of rare cell types that may drive resistance or recurrence.
By leveraging HCI, researchers can study how specific cell populations within tumors respond differently to treatments, enabling more precise and effective therapeutic strategies.
Applications of High Content Imaging in Immuno-Oncology
In immuno-oncology, the interaction between immune cells and cancer cells is critical to understanding and developing new treatments. HCI plays an important role in this domain by allowing researchers to study immune engagement, biomarker expression, and the effects of immunomodulatory compounds.
Visualizing Immune Cell-Cancer Cell Interactions
One of the unique capabilities of HCI is its ability to capture the dynamic interactions between immune cells (e.g., T cells, macrophages) and cancer cells. This is particularly useful for evaluating immune synapse formation, which is essential for effective T cell-mediated killing of cancer cells.
Case Study: Researchers have used HCI to study how immune checkpoint inhibitors (such as anti-PD-1 or anti-CTLA-4) affect the interactions between T cells and cancer cells. By quantifying parameters like T cell clustering and target engagement, HCI has helped optimize the dosing and combinations of immunotherapies.
Biomarker Identification for Immunotherapy
Biomarkers are essential for predicting responses to immunotherapy. HCI allows for the high-throughput screening of potential biomarkers by quantifying their expression patterns in response to immune checkpoint inhibitors.
Example: HCI has been used to identify PD-L1 and PD-1 expression in response to immunomodulatory drugs. These biomarkers not only guide patient selection but also aid in monitoring treatment efficacy over time.
Evaluating Cytotoxicity and Immune Activation
HCI can quantify cytotoxic effects of immune cells on cancer cells, which is essential for assessing the efficacy of therapies like CAR-T cells and NK cell-based therapies. By visualizing and quantifying cell death markers and immune cell activity, researchers can better understand the potency and mechanism of action of these therapies.
This application has become crucial in CAR-T research, where the effectiveness of CAR-T cells is evaluated based on their ability to infiltrate tumors and induce cancer cell death.
Advantages of High Content Imaging in Oncology Research
HCI offers several advantages that make it indispensable in oncology research:
Precision in Multi-Parametric Analysis
HCI allows researchers to measure multiple parameters simultaneously, capturing a more comprehensive view of cellular responses to treatment.
Data-Rich Outputs
The high-dimensional data generated by HCI provides deep phenotypic insights, enabling the discovery of subtle, complex effects that might be missed by simpler assays.
Scalability
HCI supports high-throughput screening, which is essential for testing large compound libraries and accelerating drug discovery timelines.
Overcoming Challenges in High Content Imaging for Oncology
While high content imaging has revolutionized oncology research, several challenges persist. However, innovative approaches are actively addressing these barriers to enhance its utility and accessibility.
Simplifying Data Complexity
The vast datasets generated by HCI can seem daunting. To address this, advanced machine learning algorithms are being integrated into HCI workflows. These algorithms streamline data processing and extract actionable insights more efficiently, enabling researchers to focus on strategic decision-making rather than technical hurdles.
Enhancing Accessibility with Organoid Technology
The high cost of sophisticated imaging systems has traditionally limited HCI’s accessibility. By integrating HCI with 3D organoid models, researchers are able to generate highly predictive data earlier in the drug discovery process, improving the cost-to-benefit ratio. Ready-to-use tumor organoid biobanks like OrganoidBase™ provide affordable and scalable solutions, reducing the financial barrier for smaller institutions. These biobanks offer pre-validated, patient-relevant 3D tumor models that can be seamlessly paired with HCI, minimizing the need for costly in-house development.
Addressing Technical Limitations in 3D Models
While the resolution and sensitivity of HCI systems can sometimes falter in capturing complex 3D tumor models, advancements in imaging technology are closing the gap. For instance, combining HCI with 3D adult stem cell (ASC)-derived organoids provides unparalleled depth and precision in analyzing cellular behavior within the tumor microenvironment. These organoids replicate the physiological complexity of in vivo tissues, enabling more accurate visualization and quantification of cellular interactions.
Improving Translational Relevance
Traditional 2D cell cultures often fail to mimic the intricate tumor microenvironment, contributing to the high failure rate of oncology drug candidates. The adoption of ASC-derived 3D tumor organoids paired with HCI is transforming this paradigm. These models offer greater physiological relevance, enhancing the translatability of preclinical data into clinical success.
Leveraging HCI for Comprehensive Drug Screening
By integrating 3D tumor organoids with HCI, researchers can visualize dynamic drug responses at a cellular level. This combination not only facilitates detailed profiling of drug interactions but also enables the identification of optimal therapeutic strategies for challenging, treatment-resistant tumors.
The Role of Biobanks
Commercially available tumor organoid libraries provide researchers with access to diverse and patient-relevant tumor models. Paired with HCI, these biobanks enable high-throughput screening, empowering researchers to analyze multiple compounds simultaneously and derive robust data on drug efficacy, toxicity, and resistance mechanisms.
Future Directions and Innovations in High Content Imaging
The field of HCI is rapidly evolving, with several emerging trends poised to enhance its utility in oncology and immuno-oncology:
Integration with AI and Machine Learning
AI and machine learning are being integrated with HCI to improve the analysis of high-dimensional data. These technologies allow for automated identification of complex cellular patterns, leading to more accurate predictions of therapeutic responses.
Example: AI-based image analysis can detect phenotypic patterns that correlate with treatment outcomes, enabling researchers to identify the most promising compounds quickly.
Advancements in 3D Imaging and Organoid Models
Traditional 2D cell cultures are limited in replicating the complexities of the tumor microenvironment. Recent advancements in high content imaging (HCI) are transforming oncology research by adapting this technology to 3D models and organoids. These models better mimic in vivo conditions, providing insights into spatial biology and treatment responses that are more translatable to clinical settings.
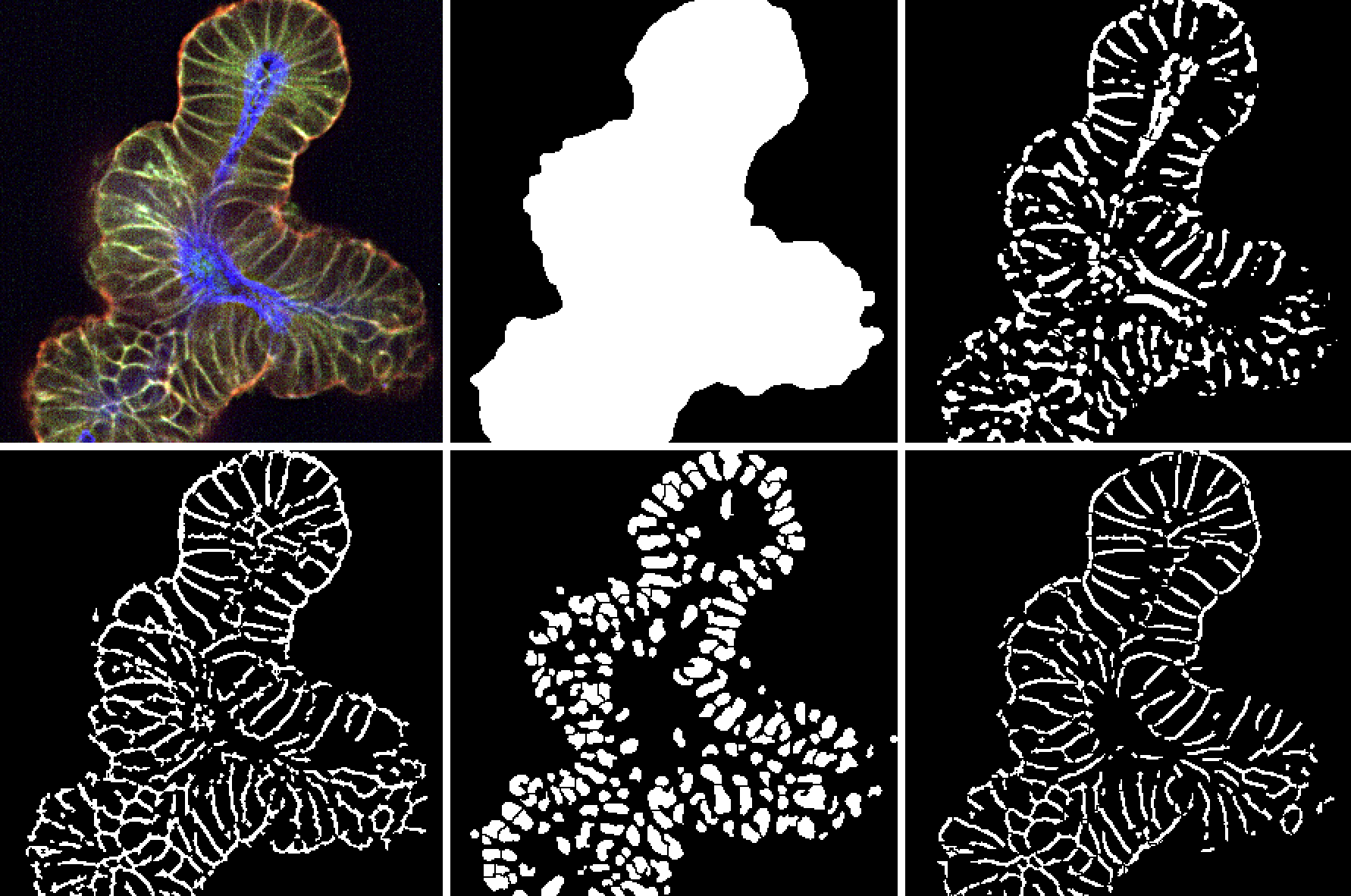
High content analysis (HCA), built upon HCI, plays a crucial role here by applying advanced algorithms to extract actionable data. When used with 3D organoids, HCA enables researchers to develop comprehensive cellular profiles, assessing treatment effects and off-target impacts in a single assay. For example, studies using patient-derived organoid (PDO) biobanks have identified novel therapeutic strategies, such as bispecific antibodies for colorectal cancer, demonstrating the practical value of combining HCI and organoids in drug discovery.
This shift toward 3D models and the integration of HCI and HCA not only improves predictive power but also addresses challenges like automation, data handling, and the need for specialized algorithms. Techniques like z-stack imaging ensure accurate visualization of complex structures, while liquid handling robotics streamline high-throughput assays. These advancements make it possible to analyze critical parameters like organoid morphology, epithelium formation, and therapy localization, providing a robust platform for oncology research.
By leveraging these innovations, researchers are better equipped to explore new compounds and therapeutic approaches, ultimately driving the future of personalized cancer treatments.
Personalized Medicine Applications
HCI holds promise for personalized oncology by allowing researchers to test patient-derived cells or organoids with various compounds, predicting which treatments will be most effective for individual patients.
As precision medicine grows, HCI could become a key tool for tailoring treatments based on patient-specific cellular responses.
Conclusion
High Content Imaging has become a cornerstone in oncology and immuno-oncology research, offering unprecedented insights into cellular behaviors and therapeutic responses. By providing a multi-parametric, data-rich view of cellular dynamics, HCI enables researchers to make more informed decisions on compound efficacy, resistance mechanisms, and immune cell interactions. As advancements in AI, 3D imaging, and organoid models continue, HCI’s potential in drug discovery and personalized medicine will only expand, solidifying its role as an essential tool in the fight against cancer.
Frequently Asked Questions