 Explore the three main types of cancer biomarkers and their main uses in cancer diagnosis, informing prognosis, and guiding treatment decisions. Review the vast array of biomolecules which act as cancer biomarkers, including genetic, transcriptomic, epigenetic, proteomic, and metabolomic biomarkers.
Explore the three main types of cancer biomarkers and their main uses in cancer diagnosis, informing prognosis, and guiding treatment decisions. Review the vast array of biomolecules which act as cancer biomarkers, including genetic, transcriptomic, epigenetic, proteomic, and metabolomic biomarkers.
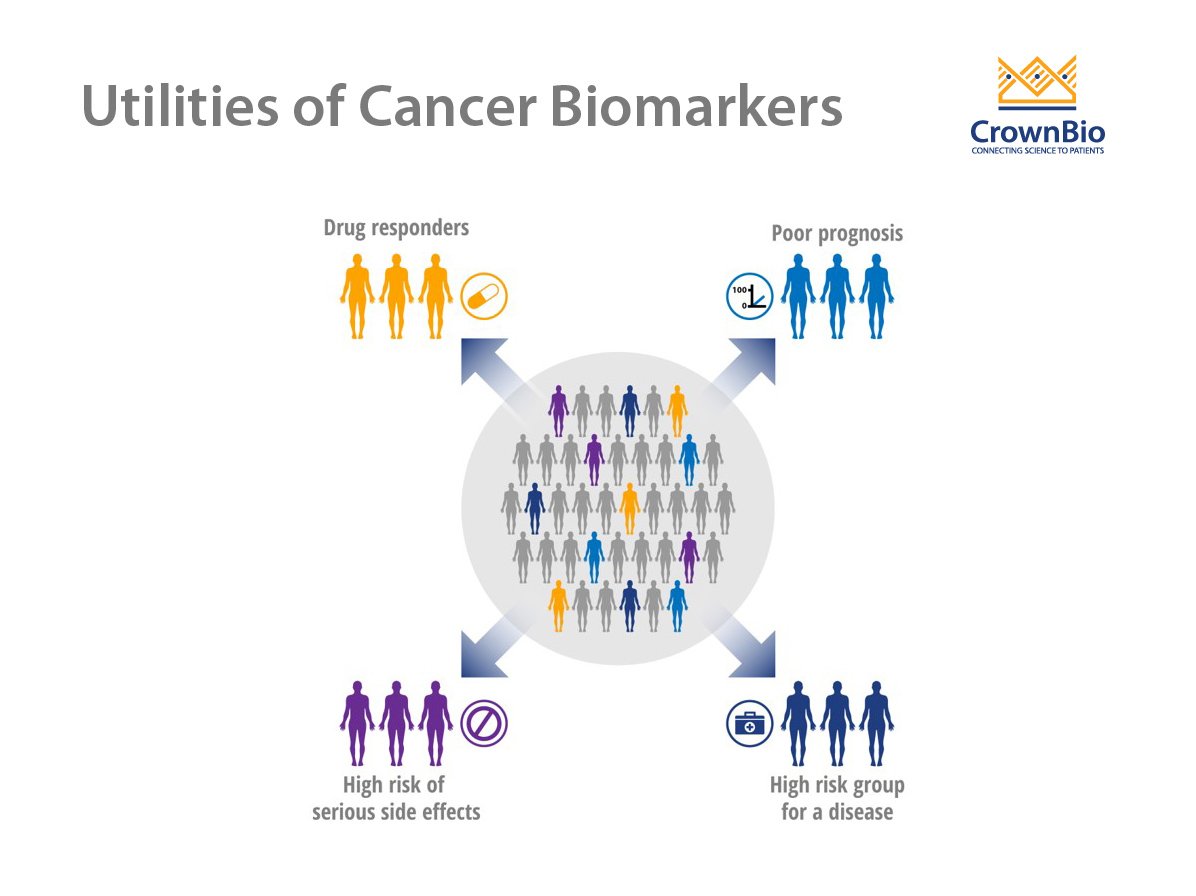
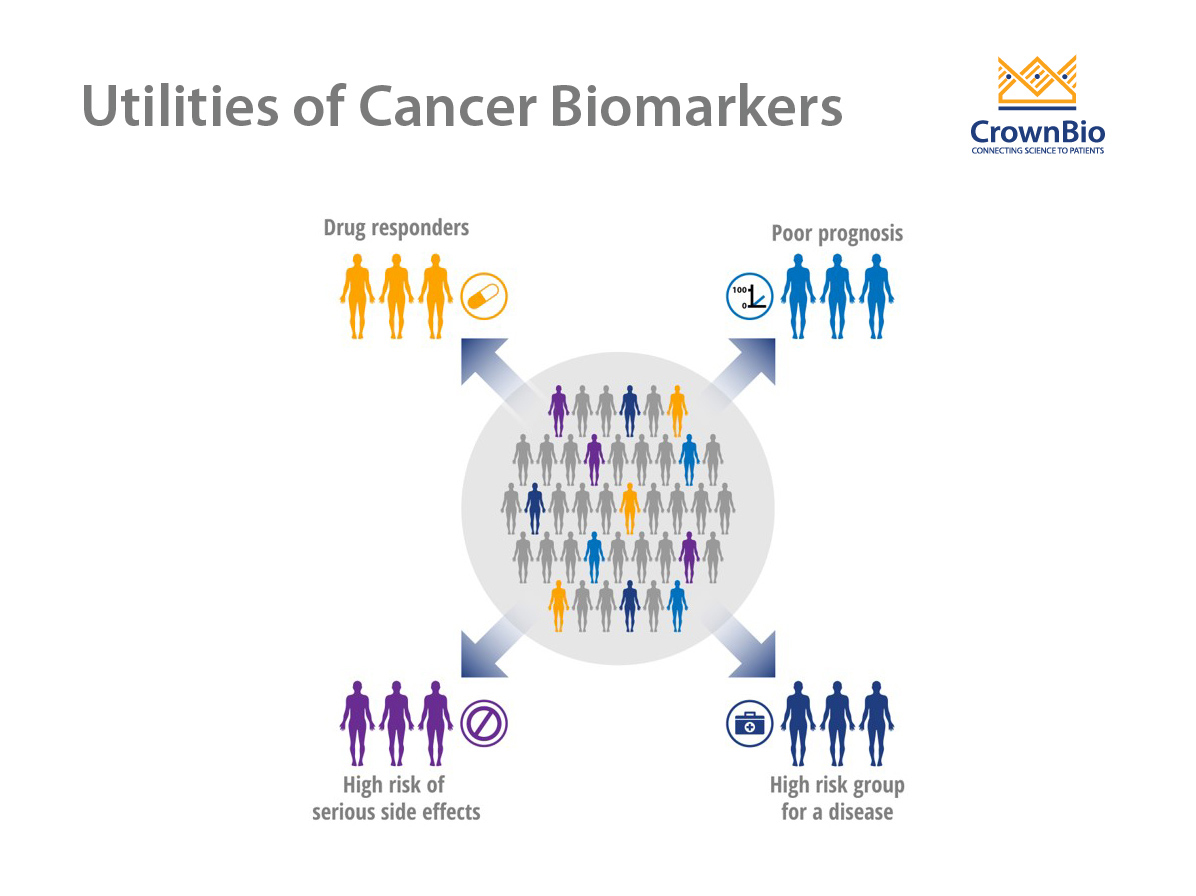
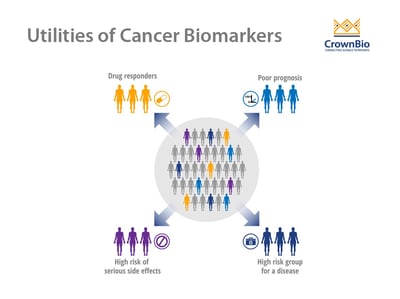
Use of Cancer Biomarkers
A wide array of potential cancer biomarkers is used to track disease development and progression or response to therapy. Biomarkers ideally suited to this purpose should be specific for a particular type of cancer and not present in normal tissues or healthy individuals. Cancer biomarkers are most often assessed by measuring the levels of biomolecules, such as proteins, peptides, biochemicals, DNA, and RNA.
This post reviews the different types and classes of cancer biomarkers along with their uses.
The Different Types of Cancer Biomarkers
There are three main types of cancer biomarkers, based on their application: predictive, prognostic, and diagnostic.
Predictive Biomarkers
Predictive biomarkers indicate the likely effect of a specific therapy on the patient and can guide treatment decisions. They include:
- HER2 positivity/activation which predicts response to trastuzumab in breast cancer.
- KRAS-activating mutations which are associated with resistance to EGFR inhibitors (e.g. cetuximab) in colorectal cancer (CRC).
- The presence of the BCR-ABL1 chromosomal alteration, which can predict positive response to treatment with tyrosine kinase inhibitors (e.g. imatinib) in chronic myelogenous leukemia (CML).
- Microsatellite instability (MSI) analysis of a tumor which provides predictive and also prognostic information. In an analysis of Grade 2 or 3 CRC patients, those with stable microsatellites or low-frequency MSI benefited from fluorouracil therapy more than those with high-frequency MSI (MSI+).
Prognostic Biomarkers
Prognostic biomarkers don’t typically trigger specific therapeutic decisions. Instead, they inform physicians about the risk of clinical outcomes, such as recurrence or disease progression. Examples of prognostic biomarkers include:
- Prostate-specific antigen (PSA) levels, if these rise over time in postoperative follow-up it can indicate local and/or systemic prostate cancer disease progression.
- Chromosome 17p deletions and TP53 mutations which predict poor survival in patients with CML.
- BRCA1 and BRCA2 gene mutations that increase the risk of breast and ovarian cancer in women and prostate cancer in men.
- Constitutive mutations in the APC gene, which predispose individuals to familial adenomatous polyposis (FAP). FAP is an inherited cancer predisposition syndrome characterized by an increased probability of polyps—and then tumors—in the digestive tract.
- Changes in DNA methylation in tumors. For example, hypermethylation of a gene promoter can lead to loss of gene expression, such as methylation of the HSBP1 gene (which encodes the heat-shock protein 27) in prostate cancer.
- The presence of circulating tumor cells (CTCs) in peripheral blood which is strongly correlated with metastasis and the formation of secondary tumor foci.
Diagnostic Biomarkers
Diagnostic biomarkers are used to identify whether a patient has a specific disease condition and are expected to have high specificity and sensitivity. They include:
- The Bence–Jones protein urine test which is often used as a diagnostic indicator of multiple myeloma.
- Elevated levels of the carcinoembryonic antigen which is used in the surveillance of CRC patients.
- Levels of PSA which are higher in patients with prostate cancer.
- CD20 which is used to diagnose and treat (via anti-CD20 antibodies, such as rituximab) relapsed and/or refractory lymphoma.
The Different Classes of Cancer Biomarkers
A wide variety of biomolecules serve as cancer biomarkers for predictive, prognostic, and diagnostic applications. These include genetic, transcriptomic, epigenetic, proteomic, and metabolomic biomarkers.
Genetic Biomarkers
Next-generation DNA sequencing technologies have facilitated the comprehensive characterization of the cancer genome and genetic biomarkers. The number of genetic biomarkers is likely to increase more over the coming years, and pan-cancer analyses are identifying mutated genes shared across cancer type subsets.
Examples of genetic biomarkers include:
- Detection of circulating tumor DNA (i.e. liquid biopsies) via blood samples provides a noninvasive method for detection and prognosis of cancer.
- BRAFV600VE mutation in melanoma predicts sensitivity to BRAF inhibitors (e.g. vemurafenib, dabrafenib, or trametinib).
- ALK gene rearrangement in lung cancer predicts response to crizotinib.
Transcriptomic Biomarkers
These are derived from the global measurement of mRNA expression, which is termed “transcriptomics.” Transcriptomics provides both measurements of single gene activity and a deeper understanding of cancer molecular subtypes. Unlike genetic biomarkers, transcriptomics is tissue specific.
Using microarray and RNAseq technologies, studies have identified panels of mRNA expression biomarkers that classify cancers into more precise subtypes based on associations with disease outcomes. Transcriptomic biomarkers include:
- KAT2B, PCNA, CD86, miR-192-5p, and miR-215-5p, identified as potential prognostic biomarkers from an analysis of cervical cancers.
- Expression of RNY3P1, RNY4P1, and RNY4P25 was significantly higher in melanoma patients with Stage 0 disease compared to healthy controls or patients with more advanced disease, suggesting a potential diagnostic utility.
Epigenetic Biomarkers
The role of epigenetics in human cancer has become an area of intensive research due to the growing understanding of specific epigenetic pathways, identification of epigenetic biomarkers, and rapid development of detection technologies. Since only 15% of cancers are thought to be hereditary, epigenetics may be key to comprehending the origin of many cancers.
Changes in the status of DNA methylation and chromatin modifications are among the most common molecular alterations in human neoplasia. In normal tissue, CpG islands (regions with a high frequency of CpG sites) of genes are mostly unmethylated. However, these CpG islands become hypermethylated in human cancers. This change represents a tumor-specific alteration in age-matched populations. As a result, DNA methylation markers can be employed in cancer diagnostics to classify and detect disease.
Abnormal methylation can predispose cells to a precancerous stage by inactivating tumor suppressor genes and cell cycle regulatory genes (via hypermethylation) and reactivating oncogenes (via hypomethylation) within the promoter region.
Examples of epigenetic biomarkers include:
- APC, GSTP1, and RARβ2 promoter methylation for PCA detection in urine and as prognostic indicators in tumor tissue samples.
- SHOX2 promoter methylation in bronchial aspirates for early lung cancer diagnosis and CDKN2A promoter methylation as a prognostic indicator.
- VIM promoter methylation in feces and SEPT9 promoter methylation (mSEPT9) in plasma for detection of CRC.
- Methylation panel made up of p14, RASSF1A, and APC, which identifies a subset of CRC patients with poor prognosis independent of tumor stage.
Proteomic Biomarkers
Proteins are the central mediators of cell function, and proteomic analyses are increasingly being combined with genomic and transcriptomic technologies to discover and validate biomarkers. Proteomic analyses provide key information about proteins’ functionality, post‐translational modifications, interaction with other biological molecules, and response to environmental factors.
Recent advances in proteomic technologies based on mass spectrometry (MS) have enabled workflows that deliver large profiling datasets with very high precision and resolution, ushering in an era where MS is more widely applied. Proteomic biomarkers include:
- CTCs which are detected according to several proteins (i.e. EpCAM, CD45, and cytokeratins 8, 18, and 19), and are useful to monitor patients with metastatic disease.
- Estrogen receptors which are used to evaluate prognosis and response to therapy.
- The risk of ovarian malignancy algorithm (ROMA) which measures the HE4 and CA-125 proteins.
Metabolomic Biomarkers
Metabolomics (also known as “metabonomics”) can be assessed in a targeted or unbiased manner, with mass spectrophotometry used to identify specific metabolites. Metabolomics is a particularly promising for biomarker development because an altered metabolism is considered a hallmark of cancer.
Understanding the metabolic alterations of tumors remains an important task. It will help to not only delineate the mechanisms of metabolic pathway changes in cancer to facilitate early detection but also predict drug responsiveness and contribute to developing novel therapeutic strategies. Examples of metabolic biomarkers with diagnostic potential in human cancers are:
- Decreased lysophosphatidylethanolamine LPE and increased ceramide in serum of breast cancer patients.
- Decreased choline and linoleic acid in serum of lung cancer patients.
- Elevated 3-hydroxypropionic acid reduced pyruvic acid (specific) in serum of gastric cancer patients.38
Biomarkers and Companion Diagnostics: The Key to Successful Precision Medicine
“Precision medicine” refers to tailoring medical treatment to the individual characteristics of each patient. Accounting for genetic, environmental, and lifestyle factors helps researchers identify the most effective approaches.
Personalizing therapy is not a novel concept. Blood typing, for example, has been used for decades to guide successful transfusions. However, informatics and information breakthroughs in genomics, such as whole genome/next-generation sequencing technologies, combined with other -omics signatures (proteomic and metabolomics), has allowed for patient stratification and the potential personalization of therapy.
To fully realize precision medicine, two components are essential:
- A targeted therapy
- A companion test to identify a biomarker
Companion diagnostics are used to identify the genes, proteins, and other signatures needed to implement a targeted therapy. This strategy allows the stratification of patients within a given disease, selecting only those who are likely to benefit.
Therefore, identifying biomarkers that can predict treatment response is critical for the success of precision therapy in cancer patients and the oncology drug development process. Companion diagnostics may also help provide additional information on treatment effectiveness or disease progression.
A major step forward in cancer biomarkers occurred in 2017 with the first-ever approval by the US FDA for a treatment based on a biomarker regardless of the cancer’s origin or location. The FDA granted an accelerated approval to Keytruda® (pembrolizumab, Merck) to treat adults and children who have unresectable solid tumors, specific genetic biomarkers (known as microsatellite instability-high (MSI-H) or deficient DNA mismatch repair (dMMR)), and a lack of satisfactory treatment alternatives or tumor progression following prior therapy.
This was followed a couple of months later by the first FDA approval of an NGS oncology panel test for multiple companion diagnostic indications. Together, these events marked an important step forward in precision medicine by ensuring the best pairing between patients and drugs.
Conclusion
Cancer biomarkers are crucial to discovering and developing novel cancer therapeutics. They are also key in clinical practice, where they have applications in risk assessment, diagnosis, prognosis, and determination of treatment efficacy and/or relapse.
Cancer biomarkers are increasingly facilitating the molecular definition of cancer. Clinicians and researchers require a comprehensive understanding of the molecular aspects, clinical utility, and reliability of biomarkers to determine whether and in what setting a biomarker is useful for patient care and whether additional evaluation is required before integration into routine medical practice. By linking therapeutics with diagnostics, biomarkers promise to play an important role in advancing personalized medicine.