Learn more about pancreatic cancer -- its associated risk factors, diagnosis, treatment and outcomes -- and current research into this understudied disease.
Learn more about pancreatic cancer -- its associated risk factors, diagnosis, treatment and outcomes -- and current research into this understudied disease.
What is Pancreatic Adenocarcinoma (PDAC)?
Pancreatic ductal adenocarcinoma (PDAC) is a lethal condition with dismal patient outcomes and an increasing incidence rate. Currently the fourth most common cause of cancer mortality in the US, PDAC is estimated to move up to second on the list by 2030.
Pancreatic cancer has a propensity to disseminate to the lymphatic system and distant organs, and around 60% of patients present with advanced disease. The presence of clinical metastases at the time of diagnosis, together with a lack of effective treatments, leads to very high mortality rates.
There are few effective conventional treatment options for pancreatic cancer and no approved immunotherapies. The limited success of immunotherapy in pancreatic adenocarcinoma is multi-factorial. However, in the majority of patients, it’s influenced by an immunosuppressive tumor microenvironment (TME) and a lack of tumor-associated neoantigens to stimulate an immune response.
With established treatment regimens, median overall survival is less than a year, with five-year survival rates only around 6%. This puts pancreatic cancer last among all cancer types in terms of prognostic outcome for patients, and there’s been no real improvement in clinical outcome for the past 30 years.
Clearly, we need to continue to develop our research methods to better understand the risk factors associated with PDAC, the biology of this disease, and to validate novel therapies for patient treatment.
What are the Risk Factors for Pancreatic Cancer?
Pancreatic cancer is typically a disease of the elderly. 90% of newly diagnosed patients are over the age of 55, with the majority presenting in their 70s and 80s. Pancreatic cancer is also more common in men than in women.
Patients with a family history of the disease are more likely to develop PDAC, with at least an 80% increased risk compared to patients with no family history. A number of other risk factors have been identified, including BRAC2 and PALB mutations, smoking, obesity, and diabetes. Excessive alcohol consumption can lead to chronic pancreatitis and this is a known risk factor for the development of PDAC.
Pancreatic Cancer Pathogenesis
Typically, pancreatic cancer develops following a series of mutations from normal mucosa, through precursor lesions, and ultimately to an invasive malignancy. The three most well-characterized lesions are:
- Pancreatic intraepithelial neoplasia (PanIN).
- Intraductal papillary mucinous neoplasm (IPMN)
- Mucinous cystic neoplasm (MCN).
PanIN is the most common precursor of PDAC. Molecular studies on resected patient tissue show that these lesions have common genetic abnormalities with adjacent PDAC. The histological profile of PanIN progression also mirrors the accumulation of mutations in cancerous tissue.
Low grade PanIN lesions have KRAS mutations and show shortened telomeres, whilst high grade PanIN and PDAC tissue show mutations in p16, p53, CDNK27, and SMAD4, and a higher rate of KRAS mutation.
Pancreatic Cancer Progression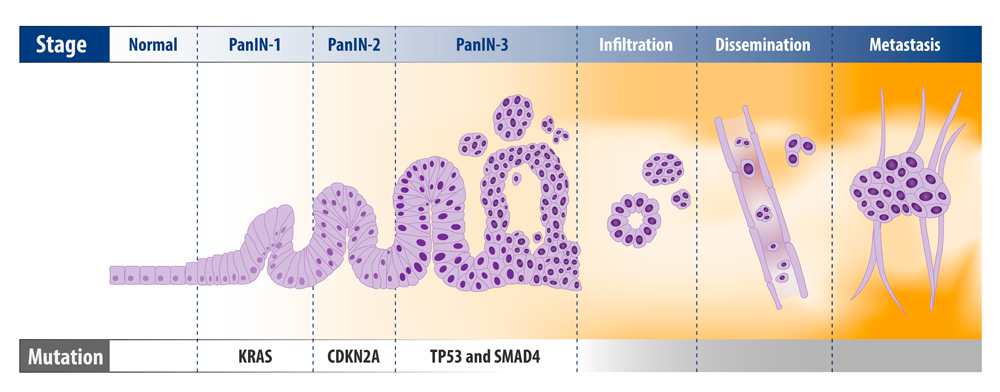
Defects in the NOTCH signaling and sonic hedgehog pathways have also been found in PDAC development. Genetic analysis shows 32 recurrently mutated genes in PDAC, which can be stratified into four distinct subgroups of disease: squamous, pancreatic progenitor, immunogenic, and aberrantly differentiated endocrine exocrine.
It’s hoped that further research into these different groups will help in the development of targeted therapies based on individual tumor biology.
Most pancreatic tumors express androgen receptors (AR) which have been the subject of intensive study for a number of years. Although androgen receptor blockade reduced cellular proliferation in pancreatic cancer in some preclinical in vitro and in vivo models, there is no current evidence of AR receptor status being of prognostic or therapeutic value clinically.
How is Pancreatic Cancer Treated?
Tumor stage at time of presentation is a major influence on patient outcome in pancreatic cancer. Typically, patients present with late stage disease, which is surgically resectable at diagnosis in only around 20% of patients. For patients who undergo surgery, five-year survival is around 20% compared to a median survival of less than a year where surgery is not an option.
Adjuvant Therapy
Adjuvant (following surgery) chemotherapy and/or radiotherapy shows some improvement in survival. The CONKO-001 study comparing adjuvant gemcitabine after surgical resection to surgery alone showed an improved median disease-free survival (13.4 months vs 6.7 months) and a five-year survival of 20.7% compared to 10.4%. However, the addition of chemotherapy only improved median survival from 20 to 23 months.
Combination chemotherapy following surgery shows some further benefit. In a trial comparing mFOLFRONIX (a combination of oxaliplatin, irinotecan, and leucovorin) to single agent gemcitabine treatment, the combination therapy showed a significantly improved disease-free survival (21.6 months vs 12.8 months) and improved overall survival (54.4 months vs 35 months).
However, mFOLFRONIX has an associated increased risk of complications. Currently, this regimen is only given to a limited set of patients with tumors of the head, body, and tail of the pancreas, whilst other patients receive a combination of gemcitabine and capecitabine.
Metastatic Disease
In patients presenting with metastatic disease, palliative chemotherapy is usually given with 5-fluorouracil added to the mFOLFRONIX regime. Clinical trials have shown an improvement in median overall survival with this regimen compared to gemcitabine treatment (11.1 months vs 6.8 months). Despite an increased risk of adverse events associated with combination chemotherapy, this is the recommended treatment for patients with metastatic disease.
Neoadjuvant therapy, where chemo or radiotherapy is administered prior to resection, has shown some success in other cancer types such as colorectal and esophageal cancers. In these cases, neoadjuvant therapy eliminates micrometastases and causes shrinkage of the primary tumor, improving outcomes following surgery.
In pancreatic cancer, however, results have been mixed. Chemo and radiotherapy have been shown to cause fibrosis within the pancreas, which can increase post-surgical complications. Work is ongoing in a number of Phase 3 trials to assess the suitability of neoadjuvant therapy as a treatment option in PDAC.
Further Research in PDAC Treatment
As we have seen there are few effective conventional treatment options for PDAC and even these do not dramatically improve disease prognosis. Systemic therapies for pancreatic cancer remain a significant unmet need.
The development of novel treatments, such as oncolytic virus therapy and immunotherapies, requires the evolution of robust models to allow us to understand and characterize the tumor microenvironment for pancreatic cancer.
It has been proposed that elevated tumor-infiltrating suppressive macrophages (TAM) and B cells have led to prevention of T cell infiltration and activation, and high levels of fibrosis. Immunotherapy with antibodies targeting PD-1, PD-L1, or CTLA-4 has not up to now shown clinical activity in pancreatic cancer.
Adoptive cellular therapy using tumor antigen-specific T cells was originally shown to have success in malignant melanoma. Further refinement of antigen specificity was achieved in the 1990s following the development of gene transfer techniques that enabled introduction of chimeric antigen receptors (CARs) into T cells.
It has been proposed that CAR T cell therapy represents an emerging therapeutic option for pancreatic cancer. Orthotopically transplanted PDAC material is believed to more accurately recapture the TME of human pancreatic cancer than conventional subcutaneous models. Recent results show that anti-mesothelin CAR-T cells efficiently inhibit the growth of pancreatic cancer patient-derived tumor xenograft models (PDX) in mice. Further investigation is warranted to elucidate this as a potential novel treatment strategy for patients with pancreatic cancer.
Conclusion
Despite many years of research, pancreatic cancer remains unresponsive to various therapies and surgical resection is only an option in around 20% of cases. Prognosis is very poor and the propensity of the disease to form metastases makes treatment very difficult.
There is a need for preclinical models which better recapitulate the human disease, to allow us to garner an understanding of the mechanisms at play. The use of more advanced models of pancreatic cancer such as orthotopically transplanted PDX models which more accurately represent the TME of human pancreatic cancer holds great promise for the evaluation of targeted therapies, and ultimately improvements in patient outcome.