Syngeneic Tumor Mouse Models: The Pros and Cons
 Immunotherapeutics are bringing remarkable survival rates for certain cancer patients, but they are also bringing some remarkable challenges for oncology drug developers. Evaluating immunomodulatory agents is more complex than assessing “common” chemotherapeutics or targeted agents. It requires preclinical models with an intact immune system (or at least functional aspects of the immune system targeted by a novel agent), which has led to the resurgence in use of syngeneic models.
Immunotherapeutics are bringing remarkable survival rates for certain cancer patients, but they are also bringing some remarkable challenges for oncology drug developers. Evaluating immunomodulatory agents is more complex than assessing “common” chemotherapeutics or targeted agents. It requires preclinical models with an intact immune system (or at least functional aspects of the immune system targeted by a novel agent), which has led to the resurgence in use of syngeneic models.
Syngeneic models were first developed over 70 years ago, far in advance of cell line-derived xenografts (CDX) and patient-derived xenografts (PDX), now routinely used for oncology efficacy studies. Syngeneic models are widely used in cancer research to study tumor immunology, test immunotherapies, and understand the tumor microenvironment. However, like all models, they have their advantages and disadvantages.
Immortalized Mouse Cancer Cell Lines, used with Same Inbred Mouse Strain
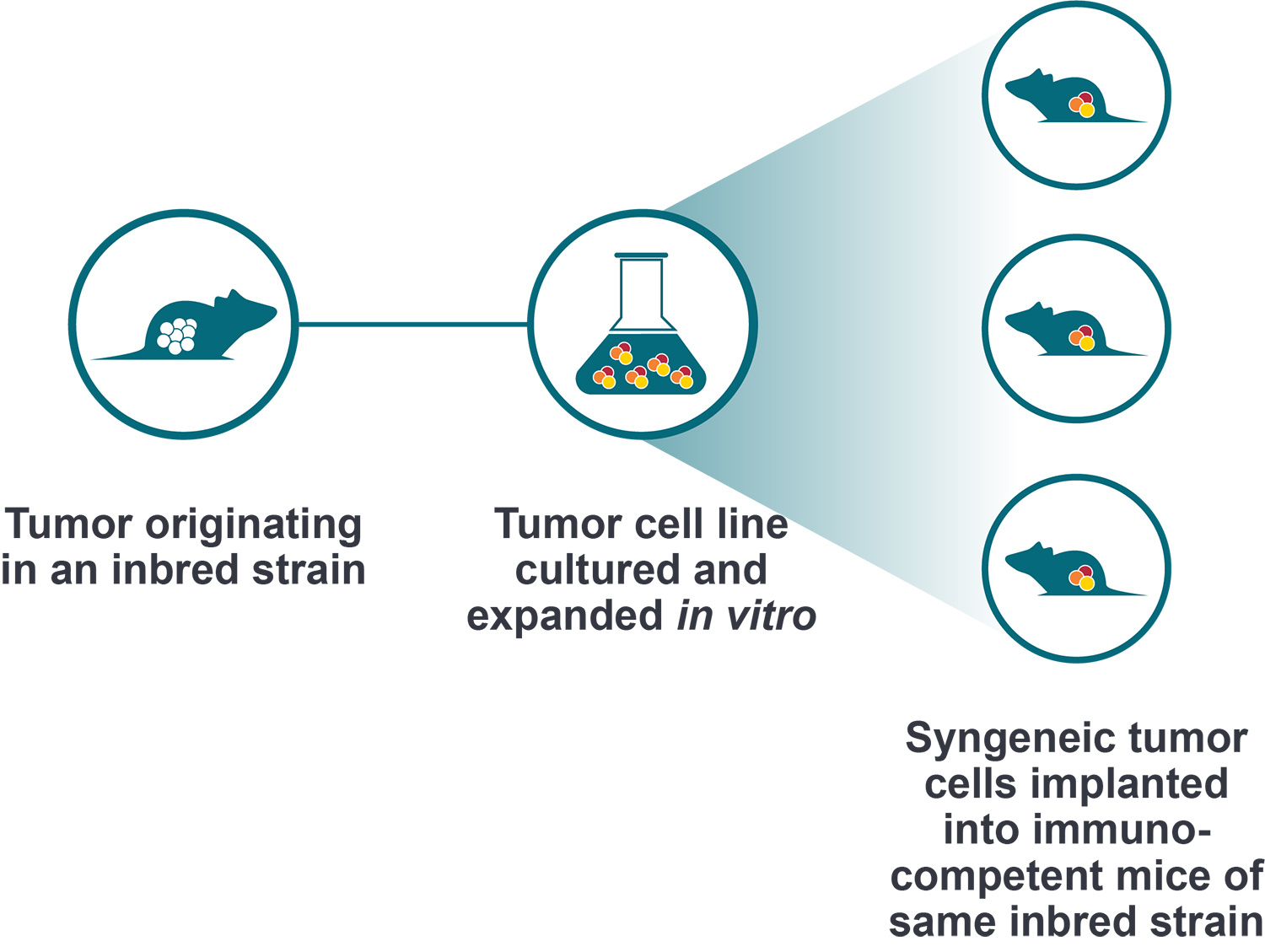
Syngeneic models are allografts immortalized from mouse cancer cell lines, which are then engrafted back into the same inbred immunocompetent mouse strain. The identical host and cell line strain means that tumor rejection doesn’t occur, creating an immunocompetent model for immunotherapy assessment.
While syngeneics used to be widely used, they were usurped by human xenografts as oncology moved towards targeting human proteins. With the continued rise of immunotherapeutics, the immunocompetent syngeneics have experienced a resurgence in their usage.
An Easy to Use, Inexpensive Model
Obviously, the main feature of syngeneic models is that they are immunocompetent, featuring full murine immunity and comprehensive stroma. Another key factor in their increased use is their relative simplicity compared with other immunocompetent models (e.g. genetically engineered mouse models (GEMMs), humanized mice).
Syngeneics are the immunocompetent model that most closely resemble running a standard allograft study. Syngeneic cell lines can be easily cultured and expanded in any lab. The models have 100% penetrance and subcutaneous injections can be carefully timed to synchronize tumor development and mimic xenograft study design. This results in short efficacy studies (potentially 2-4 weeks) performed with statistically meaningful numbers of mice per group, which are inexpensive and reasonably simple to run.
Syngeneic Uses: Ideal for Combination Therapy
Syngeneic models have a few key uses in immunotherapeutic assessment, the first being combination studies, particularly using checkpoint inhibitors. Syngeneic model panels can be extensively characterized (e.g. cell lines and tumors RNA sequenced, immunophenotyping, biomarker identification), and this data combined with in vivo efficacy benchmarking profiling results from common checkpoint inhibitors (anti-PD-1, PD-L1, CTLA-4). One of the main advantages of syngeneic models is that they maintain an intact immune system. This allows researchers to study the complex interactions between the tumor and the immune system, which is crucial for understanding the mechanisms of immunotherapies.
Taken together, these datasets provide the relevant information needed to select the right models and doses for combination therapy studies.
Fast Track Immunotherapeutics with Syngeneic Model Panels
Syngeneic model panels can also be used to fast track immunotherapies and combination regimens through large scale screening. Normally, in vitro screens are used to quickly and cheaply identify cells and models to take forward for further study. However, when you involve the complex immune system, these screens often fail, and switching to an in vivo study is usually too expensive. A large panel of well-characterized syngeneics used in a set schedule, with a shared vehicle group can fill this gap, providing a fast and cost-effective screening method.
Bioluminescent Syngeneics Available for Metastatic Modeling
Finally, orthotopic bioluminescent syngeneics are also being developed to help metastatic modeling. These models can provide real time, in-life modeling of metastatic invasion, lesions in secondary organs, and disease progression to late-stage, as well as allowing assessment of new agents to overcome this metastasis.
Syngeneic Limitations: Restricted Model Types
All models have their drawbacks, and while syngeneics are extremely useful they do have some limitations.
The number of available murine cell lines is limited. While model panels include 30+ syngeneics, this is a significantly lower number than human xenograft models. Certain cancer types (for example lung) are also underrepresented, meaning not all model types or subtypes are available.
Murine Biology Only
One feature which makes the syngeneics so useful (fully competent immunity) is also a drawback, as this immunity (as well as all biology and stroma) is that of the mouse. There can be difficulty in interpreting and predicting how a mouse immune response translates back to human immunity. Cancer is a highly heterogeneous disease, and these models do not capture the genetic and phenotypic diversity seen in human cancers.
Studies in Syngeneic Variation Ongoing
Finally, while these models are relatively consistent, it is not uncommon to experience study to study variation. This variability is currently being researched by many groups, with factors such as mouse microbiota, vivarium location, caging, feed etc. being evaluated for how they can affect and interplay checkpoint inhibitor response.
Further reading on syngeneic models and where they fit into the overall spectrum of immuno-oncology models can be found here from Li et al. Mosely et al. also published an extensive characterization of syngeneic models and their response to immunotherapy.
In conclusion, while syngeneic tumor mouse models have their limitations, they remain a valuable tool in cancer research. Their use in combination with other models can provide a more comprehensive understanding of cancer biology and treatment.




