 While viral therapy has existed conceptually for some time, it has only recently gained traction as a promising approach for cancer treatment. Here we look at how oncolytic viruses specifically target tumor cells and how to evaluate their efficacy in preclinical oncology research.
While viral therapy has existed conceptually for some time, it has only recently gained traction as a promising approach for cancer treatment. Here we look at how oncolytic viruses specifically target tumor cells and how to evaluate their efficacy in preclinical oncology research.
Oncolytic Viruses as Cancer Treatment
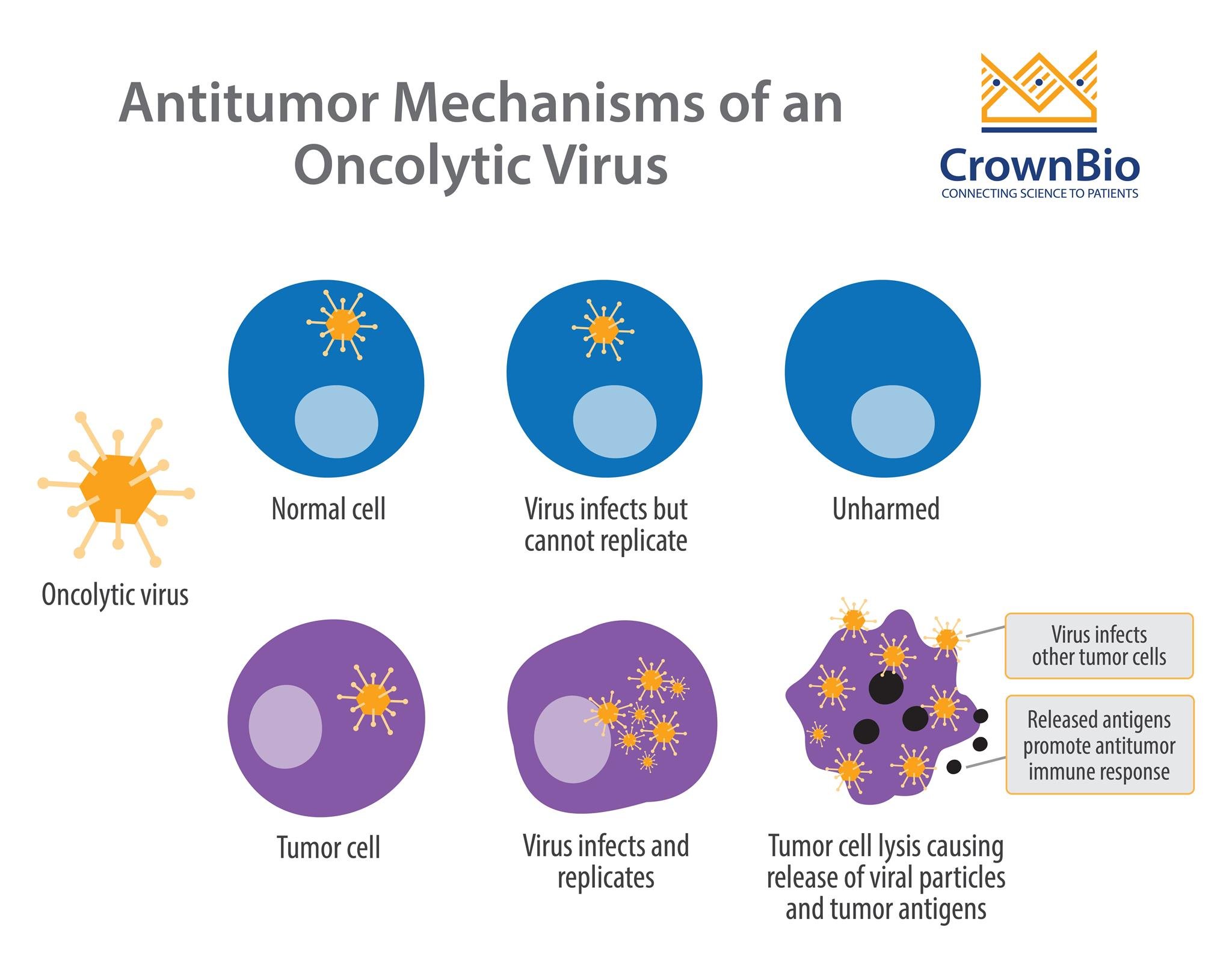
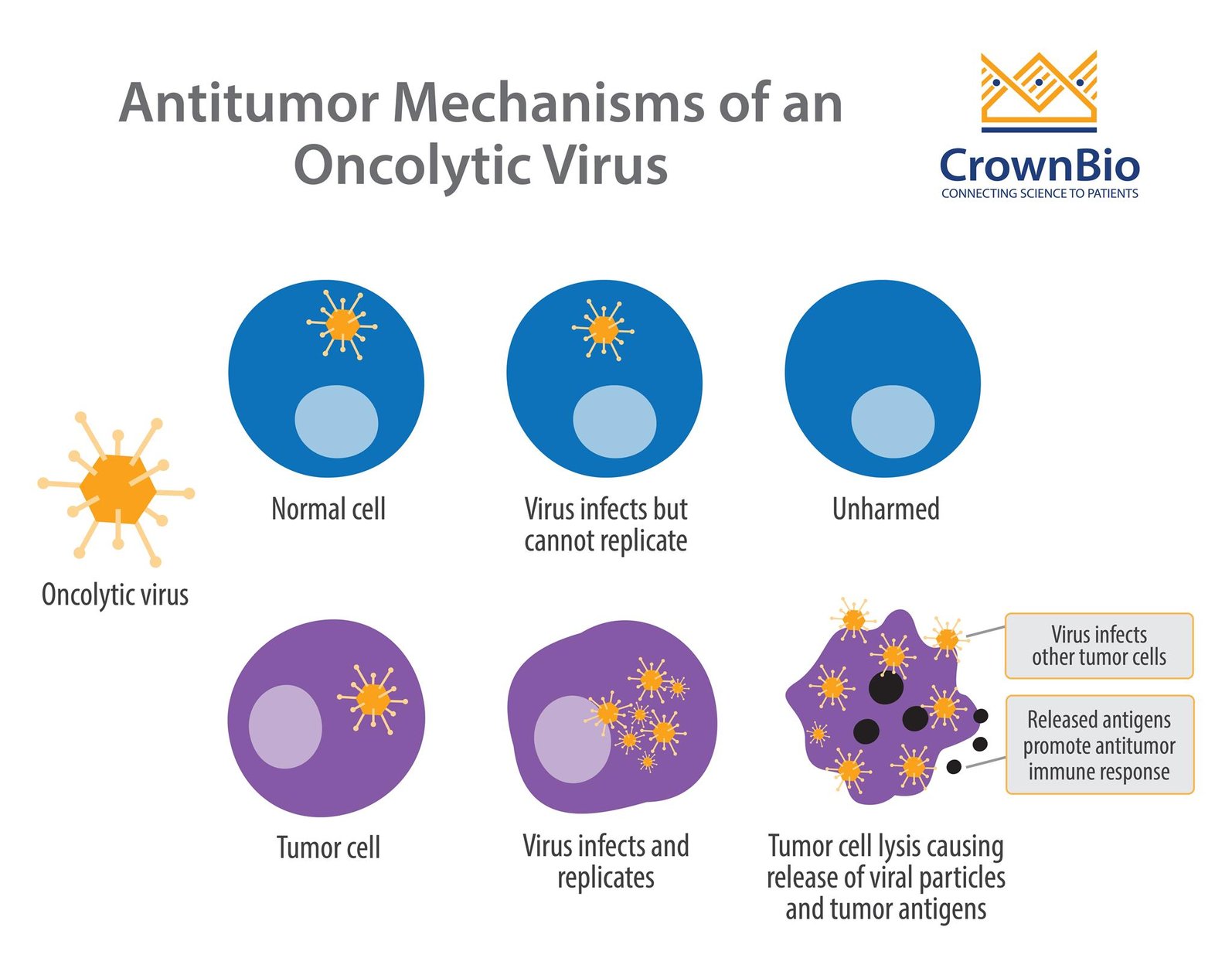
Oncolytic viruses infect, replicate, and eventually lyse cancer cells. This is the primary mechanism by which these viruses kill tumor cells. A secondary mechanism has also been proposed, whereby antigens released by dying tumor cells stimulate an antitumor immune response.
The mechanism behind viral-induced immune modulation is an active area of research. Clinical studies have so far shown that oncolytic viral therapy can enhance the number of tumor-infiltrating lymphocytes, as seen in a trial for late-stage melanoma patients.
Selectivity of Oncolytic Viruses
An important feature of oncolytic viruses is selectivity – being able to target tumor cells while sparing healthy, normal cells. Replication-selective oncolytic viruses are engineered with deletions of genes which are necessary for normal cell survival, but dispensable in tumor cells.
This attenuated form of the virus means sustained replication can occur specifically in tumor cells, due to compensatory mechanisms that the virus exploits within them.
For example, the first and only FDA approved oncolytic virus, T-VEC (Imlygic™), is a modified herpes virus with the ICP34.5 gene (the neurovirulence factor) deleted. ICP34.5 inhibits the anti-viral response pathway in normal cells, but these pathways are usually disrupted in tumor cells. Therefore, without ICP34.5, normal cells defend themselves through an anti-viral response, whereas tumor cells are missing this key defense.
Inducing Immune Response with GM-CSF
T-VEC is also engineered to contain the gene for human Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF). GM-CSF promotes dendritic cell accumulation at the site of inflammation (so the site of oncolytic activity) and antigen presentation to T cells. Adding GM-CSF to T-VEC led to regression of both injected and distant un-injected tumors, indicating a systemic immune response.
Re-implanted tumors also failed to grow in “cured” mice compared to previously non-“cured” control animals.
Together, this supports oncolytic viral therapy inducing an adaptive anti-tumor response. Clinical trials with T-VEC in combination with immunotherapy, particularly immune checkpoint inhibitors, are now ongoing for advanced melanoma.
Deletion of VGF and Thymidine Kinase to Enhance Specificity
Many viruses are also dependent on thymidine kinases, which are critical for DNA synthesis and therefore necessary for cell division. Thymidine kinases are typically overexpressed in cancer cells, due to their highly proliferative nature, and viruses with thymidine kinase genes deleted can selectively thrive within cancer cells.
Viral growth factor (VGF), produced by certain viruses, binds epidermal growth factor receptor (EGFR), creating an environment conducive for viral replication. Dual deletion of VGF and thymidine kinase can further enhance specificity in tumors that have activated EGFR/Ras signaling.
Potential Setbacks of Modified Oncolytic Viruses
Some potential setbacks to these approaches are that deleting genes which confer tumor selectivity may also reduce viral potency and lysing ability. On the other hand, these attenuated viruses also lower the pathogenicity risk for the virus handlers and reduce toxicity for animals or subjects receiving treatment.
Preclinical Oncolytic Virus Efficacy Testing
Oncolytic virus preclinical testing uses a wide variety of models, each selected to assess a specific feature or endpoint.
Xenograft Models
To measure efficacy as the primary endpoint, standard xenograft models are used in immunocompromised mice. A human cancer model is chosen to fit specific research needs, and tumor growth inhibition post-viral treatment is evaluated. This allows researchers to test their virus in a clinically translatable setting.
However, virus-induced anti-tumor immune response cannot be assessed in immunocompromised mice.
Syngeneic Models
To explore immune response alongside primary tumor growth inhibition, immunocompetent syngeneic models can be used. Dual flank studies are often performed in syngeneic mice - where one tumor is injected with virus, while the other tumor remains untreated. If both the untreated tumor and the treated tumor show regression, then this suggests a virus-induced systemic immune response.
Furthermore, adaptive immune response can be evaluated by performing re-challenge studies. In this case, tumors are implanted into animals and treated with the virus (often intra-tumorally). If the tumor regresses completely, secondary tumors can be re-implanted into the cured animal. If the new tumor doesn’t grow, this indicates an adaptive immune response.
Bio-Distribution of Viruses
The bio-distibution of viruses can also be assessed through whole mouse live-imaging, using viruses labelled fluorescently or with luciferase. This allows you to visualize which tissues the virus migrates to and replicates in. Bio-distribution of viruses in harvested tissues can also be analyzed in vitro by qPCR or immunohistochemistry.
Conclusion
Oncolytic viruses are a highly-promising therapeutic agent for the treatment of cancer. Directed engineering of these viruses allows selective tumor-killing while harnessing the immune system to fight off distant and re-occurring lesions. Optimization and testing of these viruses is an active area of research that will hopefully lead to enhanced clinical performance.