Using Syngeneic Tumor Models for POC Studies
 Syngeneic tumor models are a predictive, high-throughput option for validating novel therapeutic approaches. Here’s how to use them for immuno-oncology agent proof of concept studies.
Syngeneic tumor models are a predictive, high-throughput option for validating novel therapeutic approaches. Here’s how to use them for immuno-oncology agent proof of concept studies.
What Are Proof of Concept Studies?
Proof of concept (POC) studies aim to establish the feasibility of a novel therapeutic approach. These studies are generally conducted early on in clinical drug development, between Phase I and Phase IIa studies. POC studies are designed to test that a drug is active in a relevant system and shows evidence of efficacy in a clinically relevant endpoint.
Immunotherapy Proof of Concept Studies
Testing murine surrogate immuno-oncology agents requires a competent mouse immune system. This means that these POC studies can be carried out using syngeneic models or murine tumor cell lines within an immunocompetent host.
Why Use Syngeneic Models for POC Studies?
Syngeneic tumor models are a key platform in immuno-oncology drug development. We’ve previously discussed how to select the right syngeneic tumor model, design a meaningful study, and analyze the data generated.
The key applications of syngeneic models for immuno-oncology are Proof of Concept studies and target engagement studies to investigate mechanism of action (MOA) and pharmacodynamics (PD).
Using syngeneic models in POC studies provides a rapid, cost-effective approach to drug development with potential for high throughput. There is a wealth of knowledge and vast array of historical data available for comparison, which facilitates data interpretation. Furthermore, by harnessing the complexities of a competent immune system, any potential adverse effects involving, or amplified, by the immune system are detected early in your drug development process.
How to Use Syngeneic Models for Your POC Study
There are multiple ways syngeneic models can be used to support a POC study.
Syngeneic models are widely used to test a myriad of immunotherapy regimens. This includes:
- Single agent immune checkpoint inhibitors (ICIs).
- Combinations of multiple ICIs.
- Combinations of ICI and immunogenic cell death inducers such as standard of care agents, radiation therapy, vaccines, and virotherapies.
Microbiome Research
The role of the microbiome in immunotherapy is another area of interest that can be explored using syngeneic models. Studies have shown that the intestinal microbiota may orchestrate immune responses. Therefore, by manipulating the microbiota, we may be able to modulate cancer immunotherapy. Syngeneic models allow easy collection of gut microbes at various points of your treatment regimen for further 16S rRNA sequencing.
Bioluminescent Imaging
Because syngeneic models are derived from immortalized cell lines, bioluminescent model variants can also be generated. This allows for real time evaluation of subcutaneous as well as orthotopic tumor progression and development of metastases by bioluminescent imaging.
Immunologic Memory
For therapeutic regimens that lead to complete tumor regression (e.g. non-palpable or detectable tumors), immunologic memory can be tested by re-challenging a cured animal. A corresponding age matched control animal is also implanted with cancer cells to compare tumor growth.
Testing Combination Regimes
Last, but not least, there are some extra things to consider when choosing a syngeneic model to test combination regimens. Looking at tumor growth inhibition (TGI) as an indicator of ICI sensitivity, and at baseline immunoprofiling, can help you choose the most appropriate models for your study. Partial responder or non-responder models are particularly useful when assessing combination strategy as the performance of the combination can be measured against single treatments.
Challenges in Using Syngeneic Models for POC studies
Understanding your model is the key to effective study design, and one thing to note about syngeneic models is the inherent variability in growth and response. The majority of models, such as B16-F10 and EMT-6, behave consistently with historic data. Some other syngeneics demonstrate higher variability, such as A20, MBT-2, and Pan02, and data obtained with these models need to be carefully analyzed and interpreted.
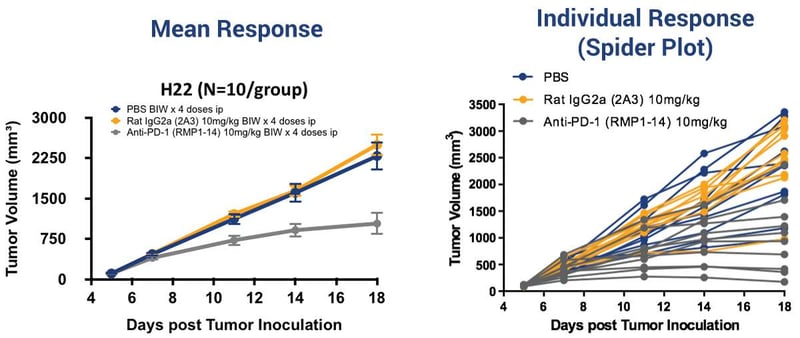
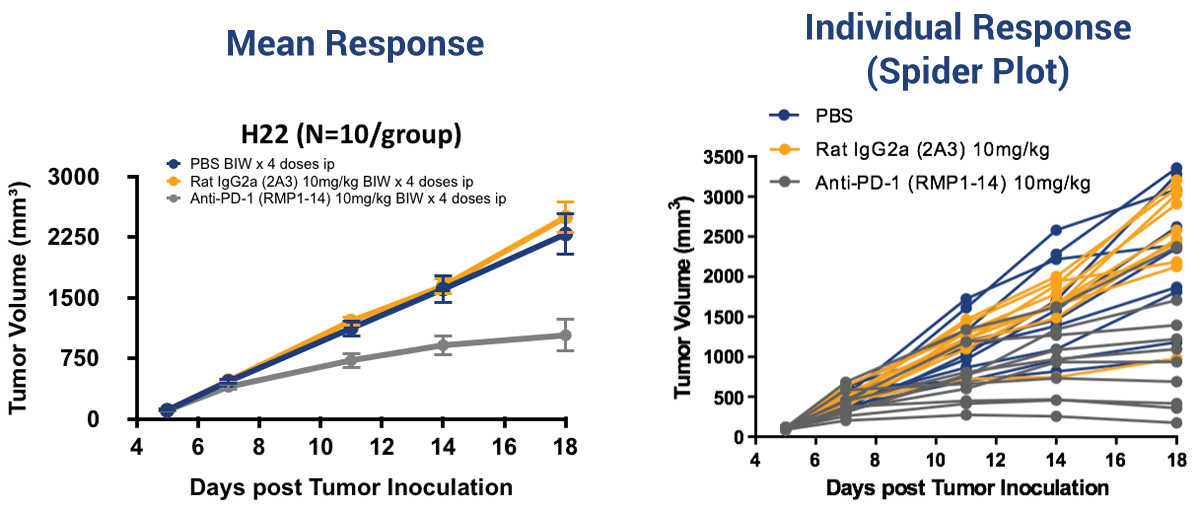
H22 Syngeneic Model Variability in Response
What makes things more complex is the intragroup variability – meaning some mice in a treatment group may respond while others may not. This is why it is important to look at individual mouse response using ‘spider plots’, shown here for the H22 model.
This variability is due to the complexity of the immune system and its interaction with the tumor in a living organism. Although mice used in syngeneic studies should all have the same genetic makeup, they each have their own unique immune signatures.
For example, mice that respond to treatment may have less myeloid derived suppressor cells (MDSCs) and upregulated levels of CD8+ effector T cells compared to non-responders. The observed intragroup variability can be exploited to look at PD markers, comparing responders and non-responders. This can provide you with an idea as to why the response may be different.
To compensate for this inherent variability, it’s important to structure a study so that you have sufficient ‘n’ per arm. This is because the statistical power increases with the number of mice. The other factor to consider is the estimated efficacy of the drug – the higher the efficacy, the less mice required to achieve statistical significance.
What Next? After Your POC Study
Overall, a thoughtfully designed POC study can set you on the path to clinical success.
Once you’ve confirmed the feasibility of your immunotherapeutic treatment regimen, the generated efficacy data can help guide the next phase of your study. A follow up study to interrogate the PD of your drug can reveal the MOA of your agent, and increase predictive power.
Additionally, you may want to select another model with specific characteristics, such as desirable growth curves and/or immune profile, to increase confidence with a reproducible response.
Cite this Article
Kato, Y., (2019) Using Syngeneic Tumor Models for POC Studies - Crown Bioscience. https://blog.crownbio.com/syngeneic-poc-studies
Related Posts


Navigating Phase 2: Preclinical Development and Optimization in Drug Discovery
Phase 2, of the drug discovery process, or preclinical development and optimization, is a critical stage in drug discovery. It bridges early lead…
Read more
Navigating Phase 1: Target Identification and Validation in Drug Discovery
Drug discovery is a journey defined by high stakes and complex challenges. From the initial identification of a target to the rigorous demands of…
Read more