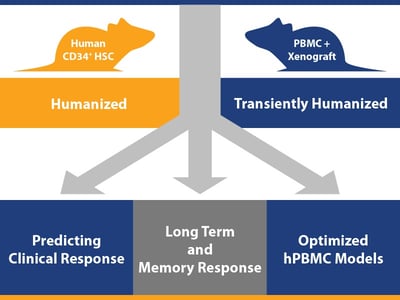
 We’ve been focusing on humanized mouse models recently on our blog, from evolution of immunodeficient host mice through to the development of derivative hPBMC and hCD34+ HSC humanized mice. This post now takes a look at how you can combine humanized mice with human tumors for three key uses in assessing human-specific immunotherapeutics.
We’ve been focusing on humanized mouse models recently on our blog, from evolution of immunodeficient host mice through to the development of derivative hPBMC and hCD34+ HSC humanized mice. This post now takes a look at how you can combine humanized mice with human tumors for three key uses in assessing human-specific immunotherapeutics.
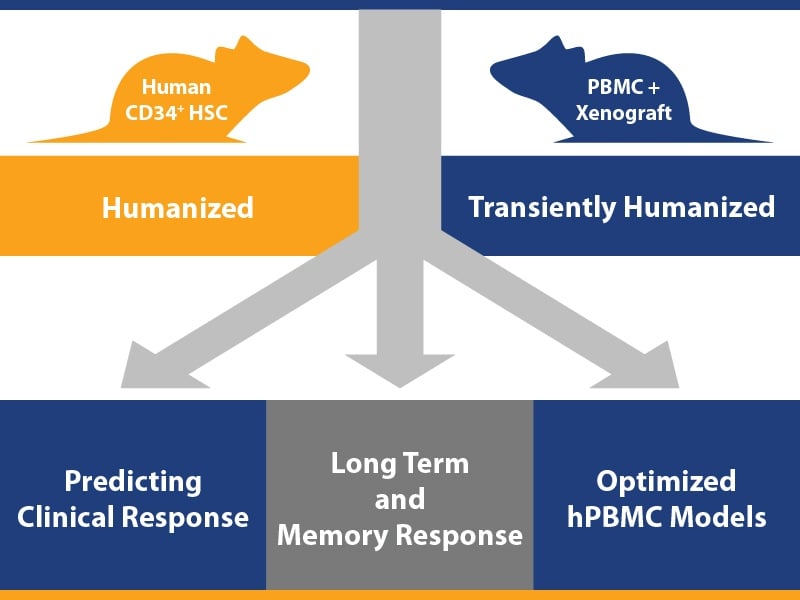
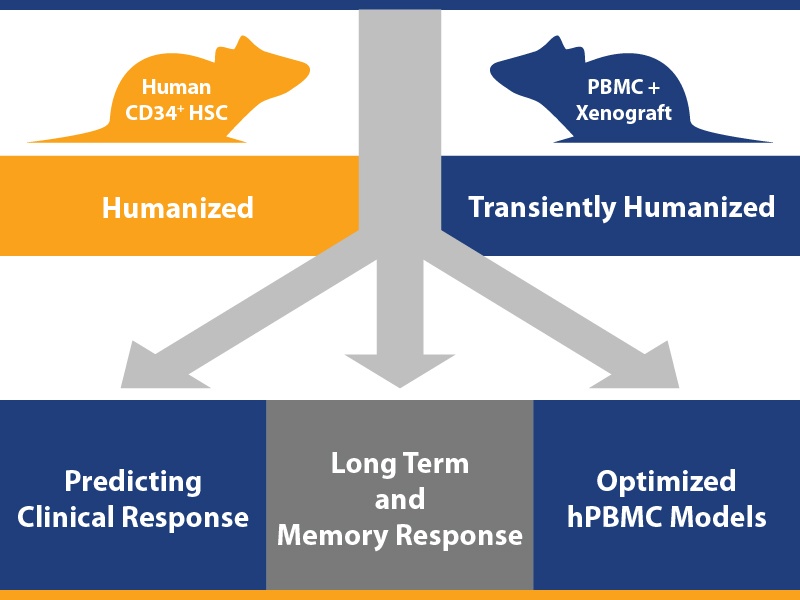
1. Effective Efficacy Studies using Optimized hPBMC Models
It’s a given that tumor bearing humanized mice are a key platform for the efficacy testing of human origin agents. The important point we are making here is the optimization of models for highly effective studies.
If you want to run a simple, straightforward efficacy test using a humanized model/human tumor combination, the easiest route is using a hPBMC model – there’s rapid immune cell reconstitution from readily commercially available PBMC resources, a wide variety of T and NK targeting agents can be tested (including checkpoint inhibitors), and it’s less costly and complex to set up than using hCD34+ HSC models.
There are 2 main drawbacks though which need to be overcome for an effective study - donor-to-donor variability and the onset of graft versus host disease (GvHD) – hence the need to optimize models.
Use Two Different Donors to Overcome Variability
Donor-to-donor variability can be partially overcome in hPBMC models with careful study design. Efficacy varies in mice from different donors, so by using PBMC from at least two different donors in each study you can work around the issue.
Optimize Each Tumor Model/Cell Donor Combination Inoculation Protocol Before Studies Begin
GvHD can also be overcome, it just requires careful study protocol planning and optimizing. hPBMC models start to develop GvHD 2-3 weeks post immune cell engraftment, with severe onset around week 4, meaning studies need to be timed just right for agent assessment within a short therapeutic window.
Firstly, cell line derived xenografts are usually selected as the human tumor source as they are fast growing (although rapidly growing patient-derived xenografts, or PDX, would also work). Next, for each tumor model and cell donor combination inoculation protocols need to be adjusted. You need to aim for a good level of immune cell reconstitution to occur at the same time as optimal tumor size to measure tumor suppression, with the perfect combination needing to occur within 15-20 days post-engraftment.
Synchronizing these factors for every tumor model/cell donor combination can result in a panel of humanized tumor bearing models across a range of cancer types for highly effective in vivo pharmacology studies such as selecting agents before moving to a fully humanized more complex study, or when working on short timelines or for high throughput studies.
2. Investigating Long Term and Memory Response to Immunotherapeutics
With long-term antitumor effect being a highly desirable treatment outcome in immuno-oncology, models are also required which can test this preclinically. The long-term disease free survival which is observed in patients following immunotherapy treatment is associated with a memory response, which also needs to be assessed and understood.
Long Term Models Required with Multi-Lineage Differentiation of the Immune System
As these studies need to run long term, GvHD rules out hPBMC models meaning hCD34+ humanized mice must be used. hCD34+ humanized mice are actually great for this type of study, with commonly used models supporting human immune cell maturation up to 32 weeks post engraftment for long-term response assessment, and multi-lineage differentiation of the immune system allowing more complex studies and more specific mechanistic questions to be answered.
Both PDX and traditional xenografts can be used for these studies, cell line derived xenografts are often selected due to their faster growth and a lack of lag time (which would need to be factored in when rechallenging models).
First Confirm Model Response, Then Assess Immune Memory with Tumor Rechallenge
Before you assess memory response, you need to set some initial baseline results – does the xenograft tumor grow well in the humanized mouse (i.e. unaffected by humanization compared with immunodeficient parental mice), does the model respond the to the test agent with tumor growth suppression, and is this response reliant on the immune system (i.e. does a humanized mouse model respond whereas a non-humanized doesn’t).
Once this is confirmed, and you have humanized tumor bearing models which have shown full tumor growth suppression, the models can be rechallenged. This involves re-inoculating the original treated cohort with another batch of cells from the same original cell line. This should be paired with also inoculating age-matched, naïve, humanized control mice with same cell line batch, to confirm malignancy of the cell line.
To observe a memory response, you would expect the age matched controls to show unchecked tumor growth, while the original cohort may have some tumor growth before this is suppressed, without further treatment, indicating a T cell memory response.
Overall, this study type confirms hCD34+ models as an excellent tool for testing long-term antitumor effect of immunotherapies, which will be highly useful as immunotherapy development continues.
3. Predicting Clinical Response Using Humanized PDX Models
A third key use for humanized tumor bearing models is in predicting clinical response in the preclinical setting, which needs to account for patient variability in response. We’ve discussed this in detail in a previous blog post – looking at checkerboard humanized Mouse Clinical Trials for advanced drug candidates.
Briefly, a population style study tests multiple tumor models across multiple stem cell donors. Each model + donor is a unique combination, resulting in a highly increased number of outcomes from just testing the range of models against one donor. This becomes reflective of a small scale clinical trial.
Population Style Study to Overcome Variability in Response and Immune Donor
These studies are important to study and overcome variability in response – which is wide ranging in the clinic – and influenced by the immunological state of each subject. Using this tailored study design (considering both numbers of animals and immune cell donors) and specifically using humanized PDX models (which more closely recapitulate patient tumors and reflect variability of response across the patient populations) overcomes limitations arising from testing an immunotherapy using a single donor, and provides a predictive preclinical setting (as well as statistically powerful results).
The results generated can be grouped by responders and non-responders, allowing discovery of predictive biomarkers of response, and for formulating patient stratification guidelines, which could hopefully help to improve patient selection and ultimately clinical success.
Many Varied Uses for Humanized Mouse Models in Immuno-Oncology
As long as there are still many open questions remaining around immunotherapy, the development of preclinical models will need to carry on improving and refining to help answer them. As a current starting point, hPBMC and hCD34+ humanized mice provide useful model platforms for solving a range of problems in preclinical immunotherapy assessment.