Antioxidants are compounds that decrease the levels of reactive oxygen species (ROS), a natural byproduct of cellular metabolism. ROS cause DNA damage, which in turn is associated with cancer. Thus antioxidants are widely marketed as dietary supplements with a variety of health claims, including the ability to decrease the risk of cancer. However, a number of studies, including some clinical trials have questioned this claim, suggesting that antioxidants may actually promote cancer development in susceptible individuals.
A study published at the beginning of 2014 in Science Translational Medicine suggested that antioxidants might have a particularly detrimental effect on lung cancer development. This research was authored by a group of scientists at University of Gothenburg in Sweden who treated with antioxidants (vitamin E and acetylcysteine) mice carrying mutations that increased their risk of developing lung cancer. The group observed that animals fed a diet supplemented with antioxidants had three times as many tumors and died twice as fast as control groups fed on a supplements-free diet.
The authors explained that the mechanism behind tumor progression in antioxidants fed mice has to do with the ability of antioxidants to reduce oxidative stress and DNA damage. While this is most of the time a favorable outcome, it should be noted that various cellular mechanisms ensure that ROS levels are carefully balanced, since ROS can also protect cells by activating defensive signaling pathways. Excessive antioxidants may interfere with this balance, potentially disrupting regulatory pathways controlled by ROS such as those downstream of the p53 tumor suppressor gene. In line with these observations, the lung cancer study found that the tumor suppressor activity of p53 was attenuated in tumor bearing mice fed an antioxidants supplemented diet.
Although this first study did not show the effects of an antioxidant supplemented diet on healthy mice, it provides evidence for a procarcinogenic role of antioxidants in populations where a higher risk of cancer already exists, in strikingly similarity to some early clinical trials data.
Antioxidants and Melanoma
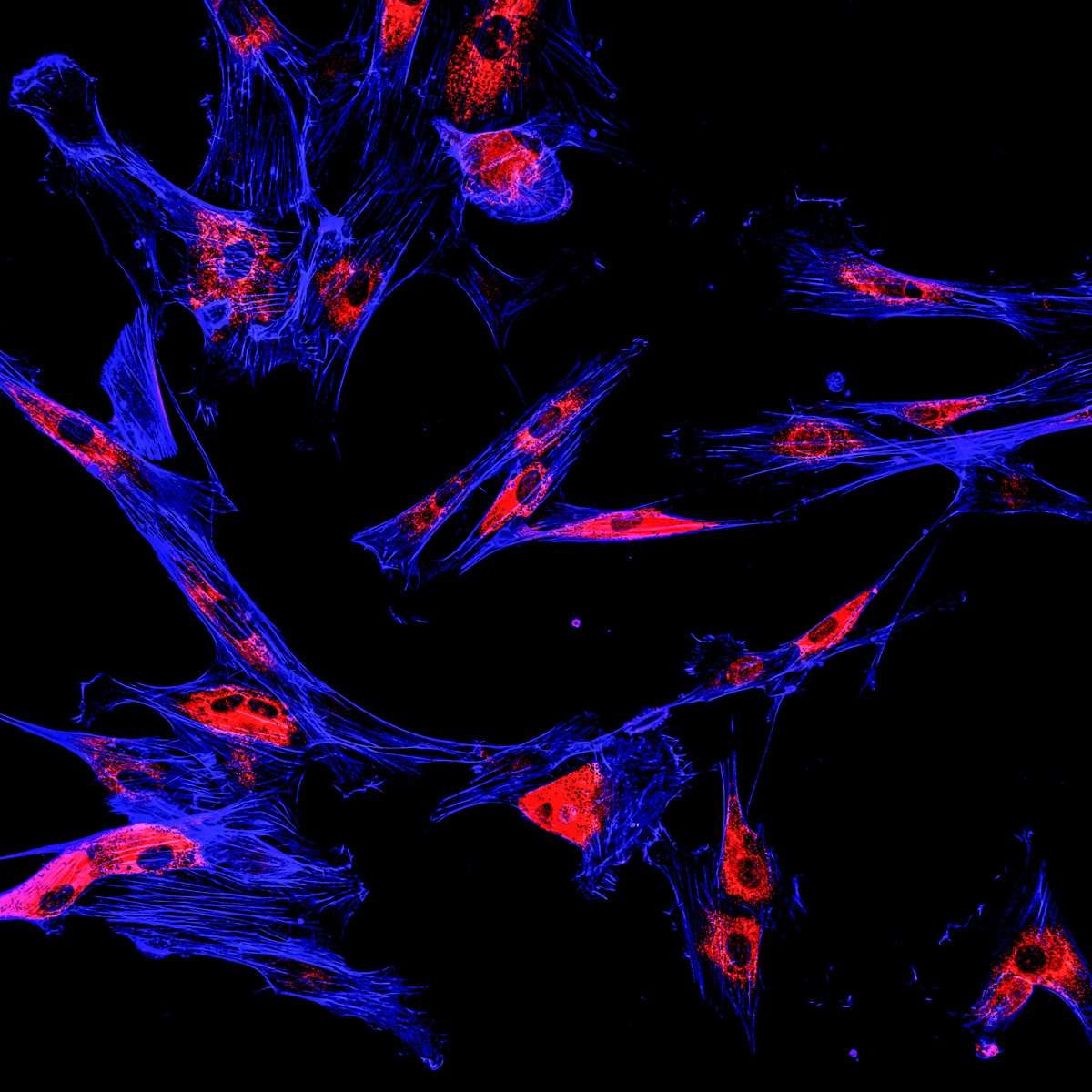
Recently the group headed by Prof. Martin Bergö experimented the effects of antioxidants (N-acetylcysteine - NAC or a soluble vitamin E analog) on cell cultures from patients with malignant melanoma. Similarly to their previous findings in lung cancer models, the researchers found that antioxidants protect healthy and tumor cells alike from free radicals, potentially enabling tumor growth. However as opposed to the lung cancer study, antioxidants added to the culture didn’t affect tumor cell proliferation, but enhanced the ability of the tumor cells to “metastasize”. These properties were dependent on the production of glutathione, an antioxidant endogenous to cells that neutralizes ROS and on the increased levels of rhoA, an enzyme activated during cell migration and invasion.
Prof. Martin Bergö and coworkers’ current research combined with information from large clinical trials with antioxidants seem to suggest that cancer patients should avoid antioxidant supplements.
At Crown Bioscience we have a longstanding track record of research in lung cancer and melanoma and we understand the importance of using the appropriate preclinical models in oncology research. Our aspiration is to help the world-wide research community turn cancer from an incurable into a manageable, chronic disease by providing the “gold-standard” collection of well-characterized models and services for drug discovery.
Our world’s largest portfolio of over 1,600 patient-derived xenograft (PDX) models, comprising the HuPrime® and PDXact™ collections, allows research into over 20 different cancer indications, including lung cancer and melanoma. Our resources further include syngenic (bioluminescent and metastatic) models, GEMM, as well as in vitro cell lines (PrimePanel™, OmniPanel™, XenoSelect™) for compound screening.
Our PDX models can be leveraged using our Translational Platforms HuTrial™, HuSignature™, and HuMark™ that allow the identification of molecular biomarkers and genetic signatures of response to stratify potential clinical trial participants, increasing the likelihood of response, and reducing anticancer drugs attrition rate.
Contact us today at busdev@crownbio.com for any further questions or information on our PDX and in vitro models and services.