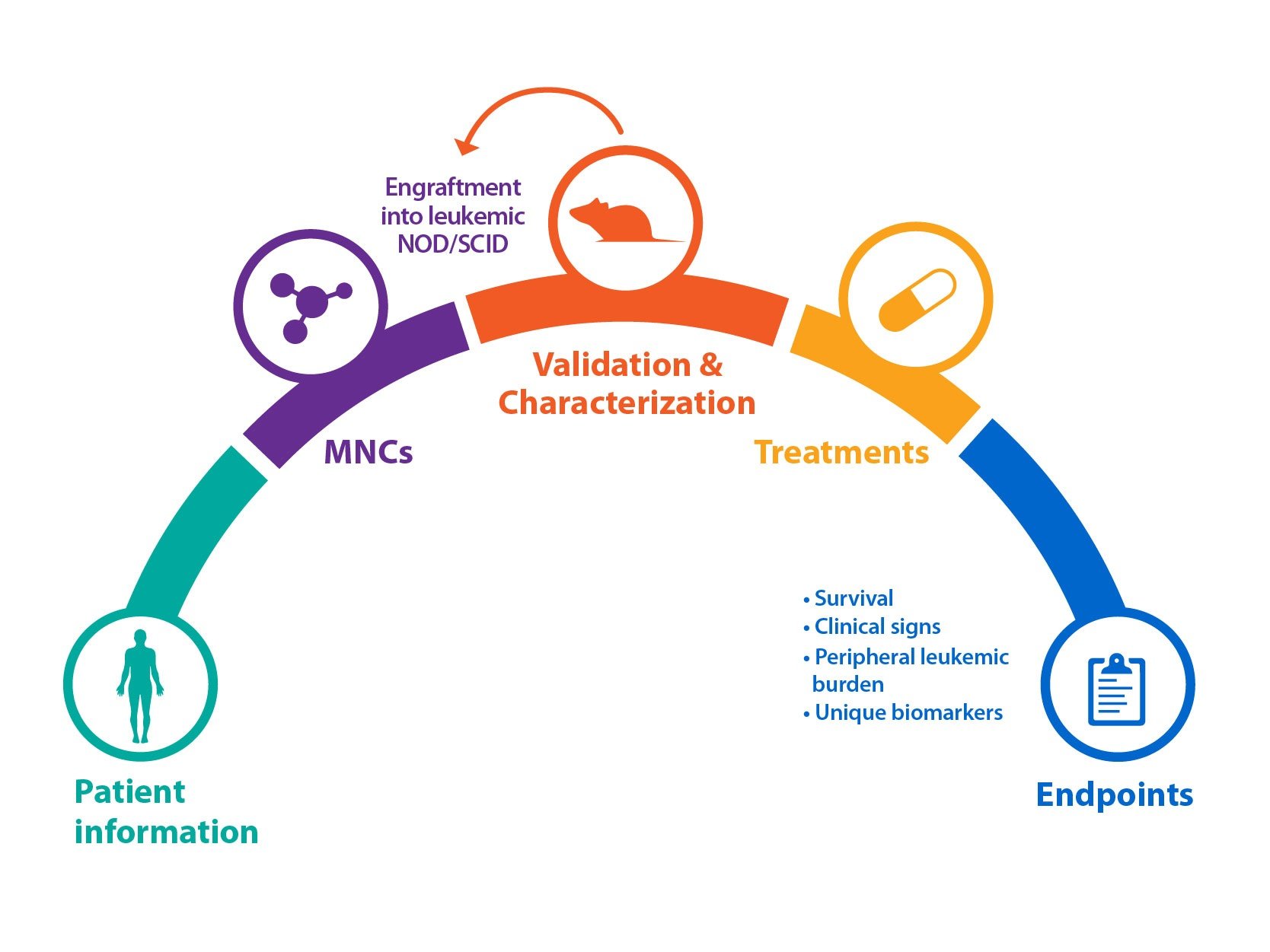
 CAR-T cell therapies are still in their infancy as clinical oncology treatments, with the first agent approved just 6 months ago. It’s known that CD19 specific CAR-T therapies induce high rates of initial response in patients, but as the treatment modality is so new, long term response studies are lacking.
CAR-T cell therapies are still in their infancy as clinical oncology treatments, with the first agent approved just 6 months ago. It’s known that CD19 specific CAR-T therapies induce high rates of initial response in patients, but as the treatment modality is so new, long term response studies are lacking.
New research published this month in The New England Journal of Medicine has followed patients from a Phase 1 CAR-T cell therapy clinical trial for up to five and a half years, to look at long term benefits of the treatment. Some interesting results have been generated, on which patients respond best to treatment, and when the therapy could be best incorporated into clinical regimens.
CD19 CAR-T Cell Therapy Trialed in Relapsed B Cell Acute Lymphoblastic Leukemia
The initial study looked at testing CD19-specific CAR-T therapies in patients with relapsed B cell ALL, who had received several rounds of chemotherapy before relapse. Of the 53 patients treated, 83% presented with complete remission; however, 14 of the patients experienced severe cytokine release syndrome (as discussed in our previous post, CAR-T therapies can induce severe toxicity and adverse events including CRS which need to be very carefully monitored).
At a median follow-up time of 29 months, the median event-free survival was 6.1 months (95% CI, 5.0 to 11.5), and the median overall survival was 12.9 months (95% CI, 8.7 to 23.4) for the whole patient cohort.
Patients with Lowest Disease Levels Showed the Best Response
The study went on to look at how disease levels affected response rate, with some very interesting results which have future implications for how and when CAR-T therapies might be implemented.
The patient cohort was divided based on disease burden – those with a “low” burden before treatment (<5% bone marrow blasts) and those with a higher disease burden (≥5% bone marrow blasts or extramedullary disease).
Patients with a low disease burden had a markedly enhanced duration of remission and survival, with:
- median event-free survival of 10.6 months (95% CI, 5.9 to not reached)
- median overall survival of 20.1 months (95% CI, 8.7 to not reached).
This compares with median event-free survival of 5.3 months and median overall survival of 12.4 months for patients with high disease burden.
Patients with Low Disease Burden also Had Fewer Side Effects
As well as an improved response, the patients with a lower disease burden also had lesser incidence of cytokine release syndrome and neurotoxic events compared with high disease burden patients.
Optimize CAR-T Cell Therapy Use: Earlier Adoption, Best Results for Patients with Less Disease
These results give an interesting insight into who might benefit most from CAR-T therapy, and when it might be most useful in a treatment schedule.
Looking at how much cancer a person has before giving CAR-T cell treatment, might be a good gauge of how that patient will respond, and which groups of patients will/will not respond well to the treatment (and therefore who might benefit more from any other available options).
Secondly, these data suggest that CAR-T might be better implemented earlier in a treatment schedule, before patient disease burden becomes too high (potentially following first-line chemotherapy) for the best long term benefit. Current approvals for CAR-T therapies are for patients who are in secondary or later relapse, by which time disease burden may be higher than liked for CAR-T treatment.
As more long term studies read out on this new modality, we’ll gain more information on how to optimize use, and provide the largest survival benefits for the greatest number of patients.
Further Reading:
- Park JH et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med 2018;378(5): 449-59.
- Almåsbak H et al. CAR T Cell Therapy: A Game Changer in Cancer Treatment. J Immunol Res 2016; 2016: 5474602.
- Healio HemOnc today. Will CAR T cells always be reserved for late lines of therapy? November 2016.
- Ruella M and Kenderian SS. Next-Generation Chimeric Antigen Receptor T-Cell Therapy: Going off the Shelf. BioDrugs 2017; 31(6):473-81.
- Ramello MC et al. CAR-T cells and combination therapies: What's next in the immunotherapy revolution? Pharmacol Res 2017; pii: S1043-6618(17)30600-X.