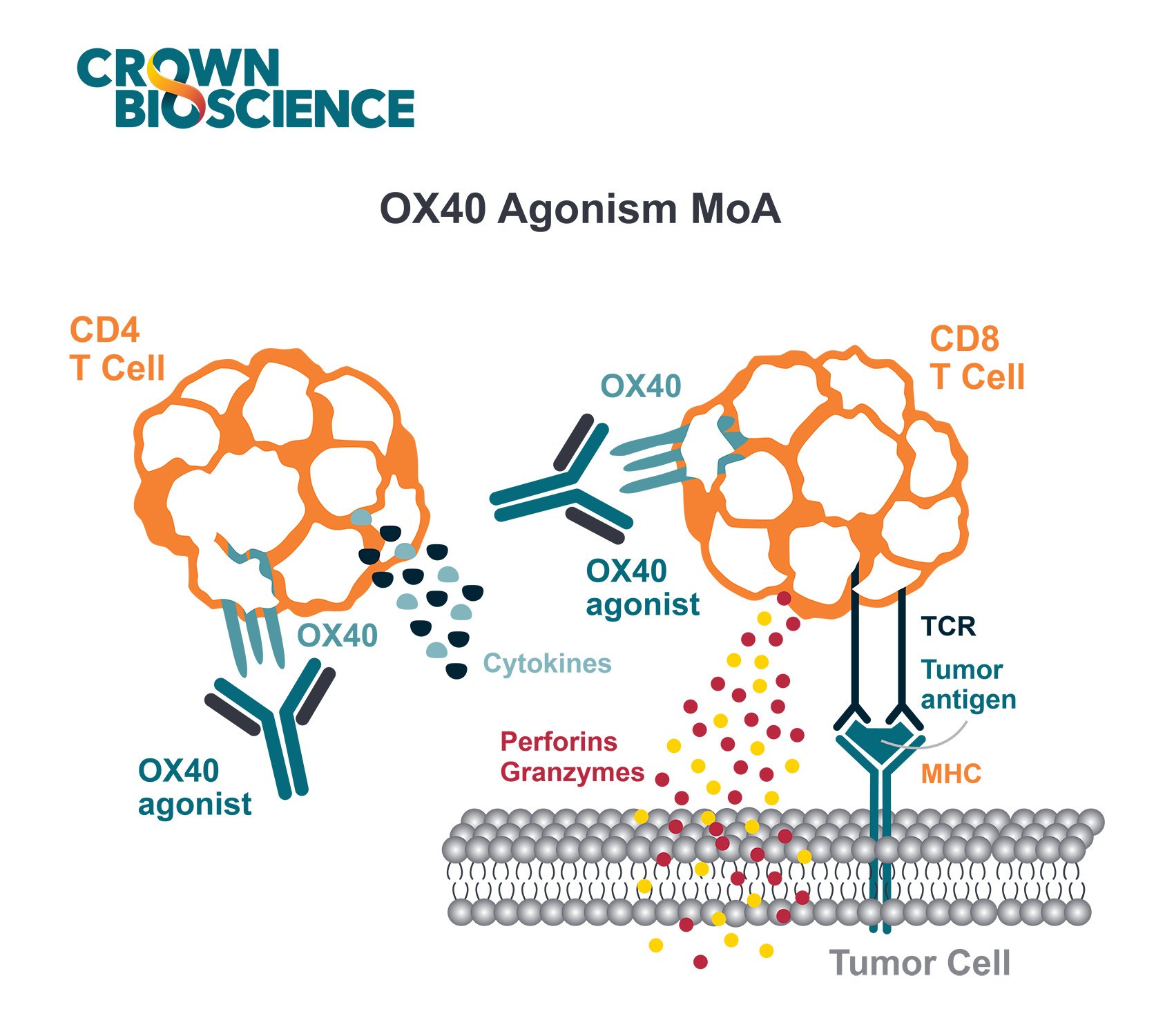
 Patient-derived xenografts (PDX) are a commonly used preclinical drug development tool, engrafted into immunodeficient mice and used to test the efficacy of novel anticancer agents. It’s widely accepted that these models provide the most predictive preclinical data to inform on clinical trial, as well as offering highly specific patient-relevant mutations, fusions, etc for targeted agent evaluation.
Patient-derived xenografts (PDX) are a commonly used preclinical drug development tool, engrafted into immunodeficient mice and used to test the efficacy of novel anticancer agents. It’s widely accepted that these models provide the most predictive preclinical data to inform on clinical trial, as well as offering highly specific patient-relevant mutations, fusions, etc for targeted agent evaluation.
As many researchers now look to these models to assess immunotherapies (or combination immunotherapy regimens), we take a look at the best way to use PDX when a functional immune system is also required...... or is it?
Assessing Immunotherapeutics in Non or Partially Immunocompetent Hosts
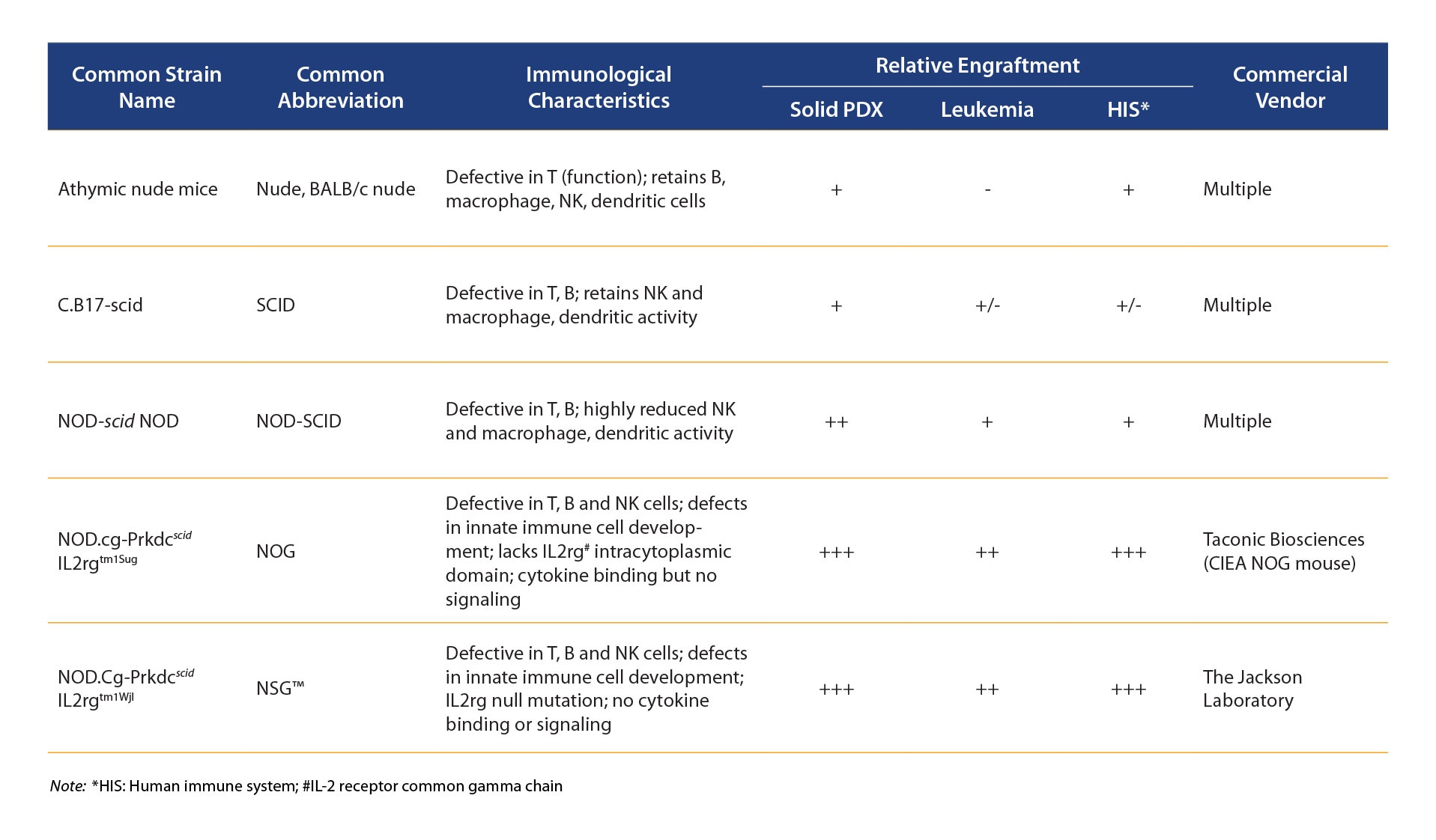
It seems counter intuitive, but the first way to use PDX to assess certain I/O agents is actually in immunodeficient or only partially immuno-competent hosts (commonly used immunodeficient mouse strains are reviewed in the table below).
Characteristics of Immunodeficient Mouse Strains
A partially immunocompetent mouse would need a lack of T cell histocompatibility function to prevent tumor rejection. Using these models types, you can evaluate specific therapeutics in which exogenous immunity can be directly introduced, for example:
- Passive immunization
- Antibody drug conjugates
- Certain cell therapies like CAR-T treatments.
Or the partial, mostly innate immunity, in mice can be used as a therapy, for example, the host mouse NK functions.
For instance, many xenografts grow in athymic nude mice with partial immunity, lacking T-cell function. Advantages of these models are simplicity, without needing humanization of mice, whilst also allowing evaluation of many human-specific biological agents compared to murine I/O models. Using PDX in this situation gives all the advantages of normal PDX studies for closely mirroring patient disease histo and molecular pathology, reflecting tumor population diversity, as well as providing predictive SoC response.
Using Human Tumors in Humanized Mice
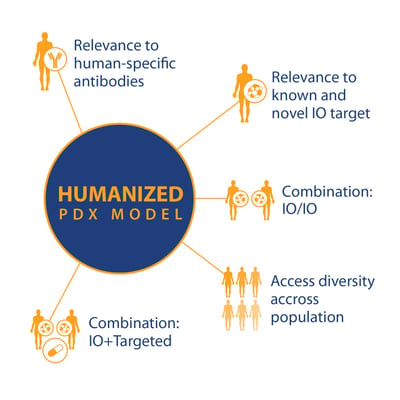
The alternative way to use PDX is as human tumors within a human immune system, therefore more accurately reflecting human immunity and disease. There are different ways to create a humanized model, and many humanized mice available from a range of vendors which support engraftment and growth of PDX models.
PDX in humanized mice are being touted as the future of I/O modeling; however, two key questions at least still need to be answered:
- Whether functional human immunity in humanized mice can mimic human immunity, at least in the context of I/O investigations
- Whether the interactions between the immune system and tumor xenografts represents a specific anti-tumor immune response, an allogeneic response, or a combination of both responses.
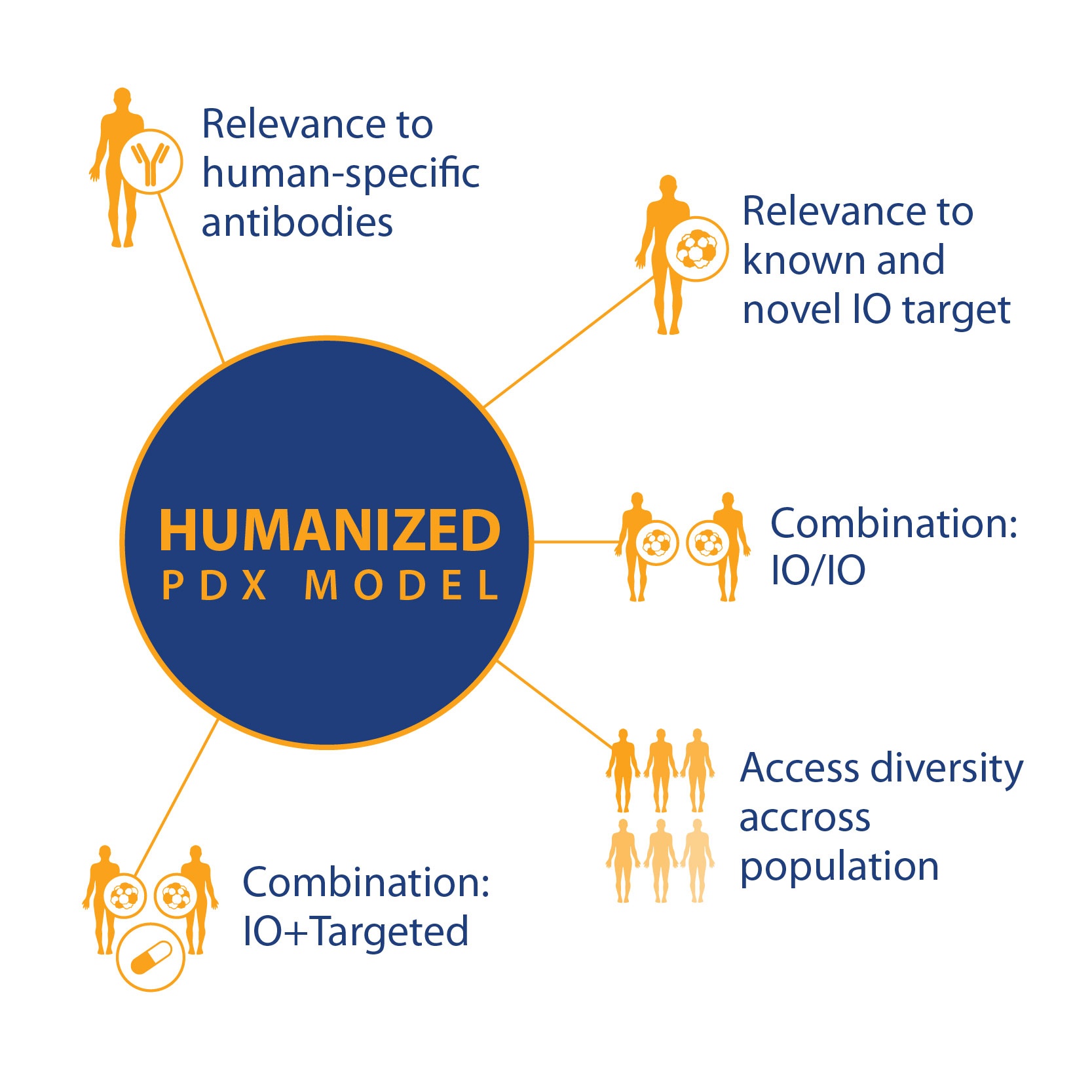
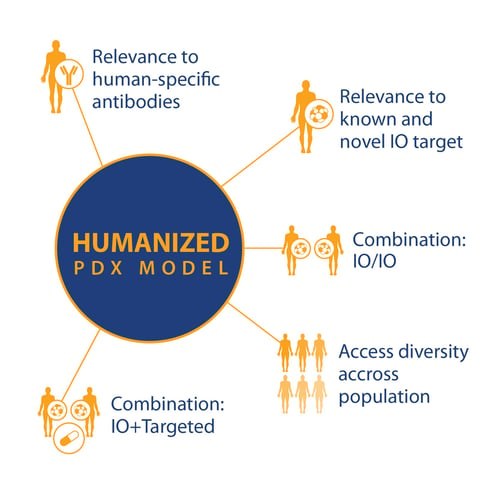
If these and other research questions can be answered, then PDX in humanized models can become a powerful tool, with a wide variety of uses based on standard PDX key features and
- Relevance to known and novel I/O targets
- Relevance to human-specific antibodies
- For I/O and combination I/O studies
- And population studies
Further reading: for a more thorough discussion on PDX uses in I/O studies read Experimental animal modeling for immuno-oncology, an overview of the wide range of models available for I/O studies today.