Blood Cancer PDX Models: Focus on Acute Myeloid Leukemia
 A number of our previous posts have covered the benefits of patient-derived xenografts (PDX), usually focusing on models derived from solid tumors. But these highly predictive preclinical models can also be developed from blood cancer.
A number of our previous posts have covered the benefits of patient-derived xenografts (PDX), usually focusing on models derived from solid tumors. But these highly predictive preclinical models can also be developed from blood cancer.
Acute myeloid leukemia (AML) is the most common adult acute leukemia. Most general treatments are chemotherapies, which can cause remission but do not cure the disease. A lack of predictive experimental AML models has been a major issue in both understanding leukemogenesis and in developing effective new treatments.
This blog post provides an introduction on AML PDX model development and what to expect from an AML model panel.
How Do You Make an AML PDX Model?
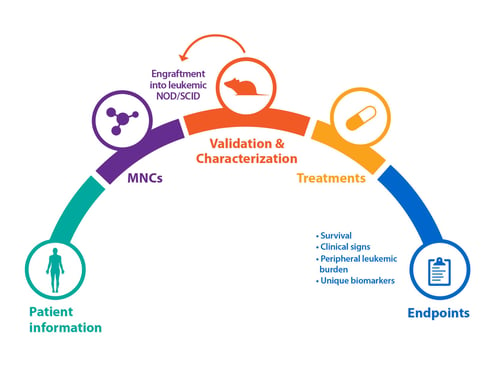
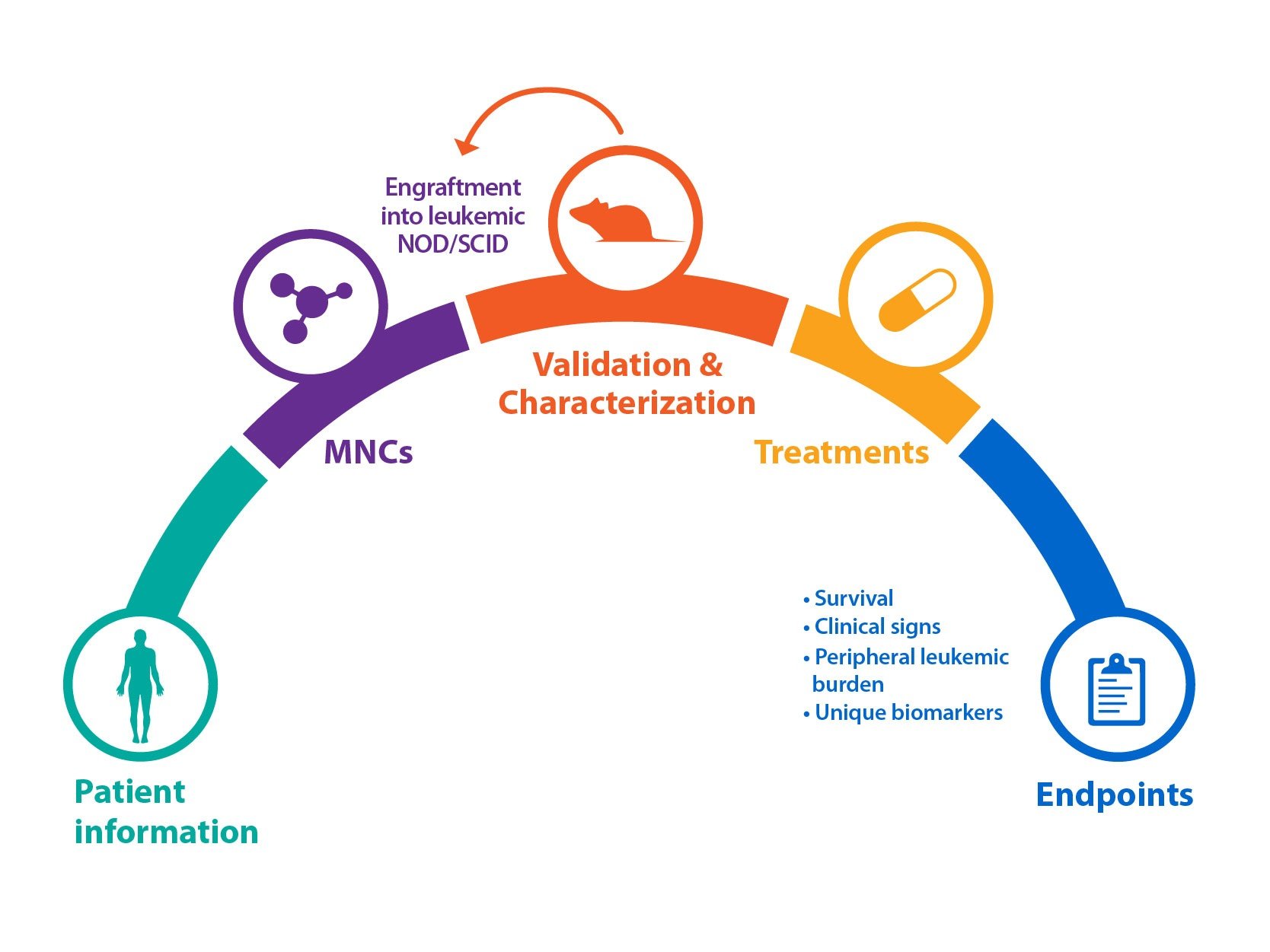
Bone marrow specimens are obtained from patients diagnosed with AML according to global, official classifications. Mononuclear cells (MNCs) are then isolated and engrafted into irradiated mice, for example NOD/SCID. Animals can then be monitored weekly for the peripheral appearance of leukemic cells (CD45+) via flow analysis, which can occur quickly (approx. 4 weeks), or after a longer latency period.
Cells from this original P0 mouse can be serially passaged via re-engraftment into recipient mice to create stable AML models. Model cells can also be kept frozen in liquid nitrogen, and removed and used as required.
Full Background Information and Characterization Available with AML PDX Models
As for solid tumor PDX models, blood cancer and AML PDX can be fully characterized and data stored within online databases for easy selection of models.
Firstly, patient background information will be collected – for example age, gender, ethnicity, and disease subtype, with a range of subtypes included within a diverse PDX collection. Information such as primary blood test results, primary marrow morphology, and patient treatment and their response is also collected.
Patient immunologic phenotype information and mutations are recorded, which can then be compared with PDX model antigen expression (determined by flow cytometry) and genetic analysis (via RNA and whole exome sequencing) to confirm the original disease is replicated.
Variety of AML PDX Phenotypes Available with Differently Differentiated Disease
Dependent on the model subtype and PDX disease, a variety of AML phenotypes can be observed in available models. This includes models which are not very differentiated and mostly limited to the bone marrow, and PDX which show more differentiated disease, moving into peripheral organs and with features such as enlarged spleens.
Immunohistochemistry can be used to show infiltration of hCD45 positive cells into a range of organs such as bone, spleen, lung, and liver.
Range of Mutations of Interest Required for Targeted Agent Development
As AML treatments move toward personalized medicine and targeted therapy, models are needed which cover the common mutations found in the clinical AML environment. Historically, it was thought somatic genetic changes contributed to AML leukemogenesis through a “two-hit” approach:
- Class I mutations cause activation of pro-proliferative pathways
- Which must occur with Class II mutations which impair normal hematopoietic differentiation and allow leukemia to develop
Common Class I mutations include(1):
- FLT3 (internal tandem duplications, ITD, and tyrosine kinase domain mutations, TKD): ~28% cases
- K/NRAS: ~12%
- TP53: ~8%
- c-KIT: ~4%
Common Class II mutations include(1):
- NPM1: ~27%
- CEBPA: ~6%
A third class of mutations has also recently emerged with downstream effects on both cellular differentiation and proliferation, including mutations in epigenetic modifiers e.g. IDH1, IDH2, and DNMT3A.
A range and combination of these mutations across PDX models to recapitulate the clinical landscape is required for full development of next generation AML targeted agents.
Models Need to be Truly Representative of the Human Condition
Historically, some commercially available models were transient in nature, and non-transferable through passages, lacking disease symptoms or mortality. While these models are useful to provide a gross measure of response, they are a limited, finite banked source from patients, and not renewable, therefore only allowing one shot studies.
Serially transplantable models have been developed and published which show typical leukemia symptoms and eventual mortality, meaning they are more representative of the human condition for preclinical drug development. These are required for primary and relapsed disease to allow study into all aspects of AML.
Model Validation is Always Needed
As for any solid tumor PDX, it should also be noted for AML models that stringent validation and quality control is important, usually via genetic fingerprinting by STR genotyping, and also monitoring robust and consistent growth over multiple passages.
While this is not an exhaustive list of the key features of AML PDX, it should help as a starting point for anyone new to the field looking to understand why AML PDX are highly useful for preclinical drug discovery.
References:
Cite this Article
Barbeau, J., (2017) Blood Cancer PDX Models: Focus on Acute Myeloid Leukemia - Crown Bioscience. https://blog.crownbio.com/blood-cancer-pdx-acute-myeloid-leukemia