 Around 95% of new anticancer drugs eventually fail in clinical trials, despite promising results when tested on 2D in vitro preclinical models. New models that better mimic tumor biology in vivo can facilitate more efficient and translatable drug discovery research.
Around 95% of new anticancer drugs eventually fail in clinical trials, despite promising results when tested on 2D in vitro preclinical models. New models that better mimic tumor biology in vivo can facilitate more efficient and translatable drug discovery research.
This post looks at advanced 3D in vitro models, including tumor spheroids and organoids.
Why Aren’t 2D In Vitro Models More Translatable?
Currently, a high proportion of drug discovery is performed using cell cultures. This increases the speed and efficiency of preclinical oncology research, but only so far as those results translate into the clinical environment. Culturing cells on flat plasticware results in artificial 2D monolayering. Cells suffer additional stress as they spread and flatten on the plastic surface, and they don’t engage in efficient cell-cell and cell-matrix interactions.
All of these factors influence gene expression and protein function. In addition, cancer cell lines accumulate genetic aberrations with increasing passage numbers. Combined with positive clonal selection, which is highly sensitive to culture conditions, this leads to cell line heterogeneity.
This raises the question of whether 2D cell culture models represent the corresponding in situ tumor well enough. And if not, is this leading to poor in vivo translation.
Is there a better way to facilitate oncology drug discovery in vitro?
3D In Vitro Models Can Be More Effective
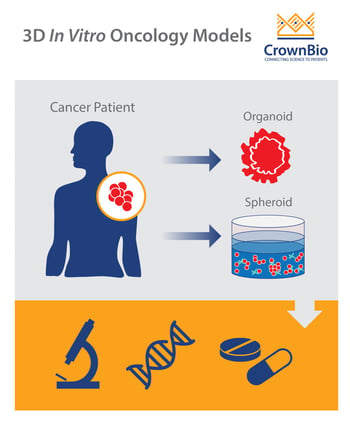
More recently, 3D cell cultures have been developed to more closely mimic in vivo heterogeneity, native histologic architecture, and response to therapeutics.
The term ‘3D cell culture’ refers to both spheroid and organoid 3D structures. Although the terminology has been used interchangeably, there are notable differences between these two techniques.
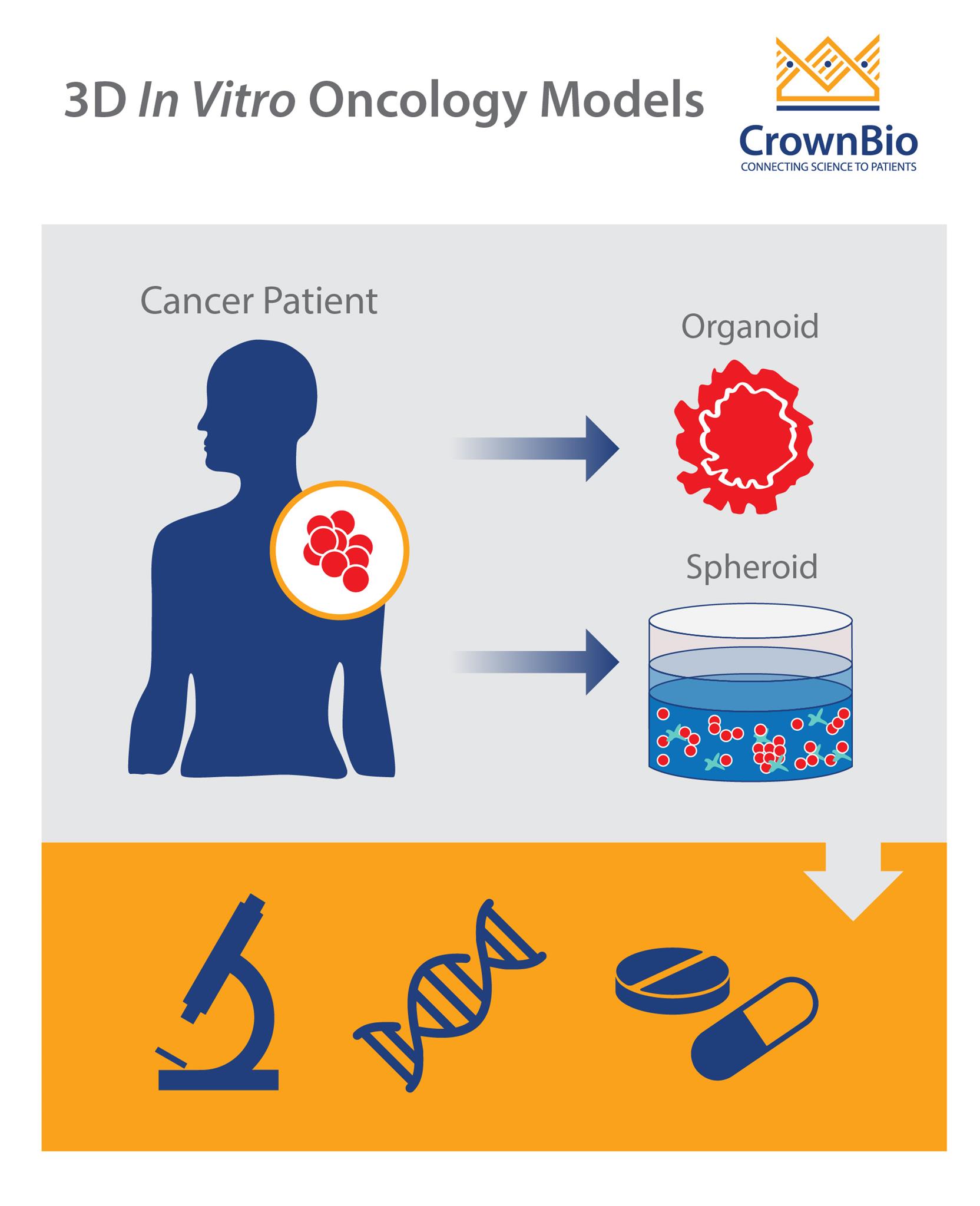
Tumor Spheroids
Tumor spheroids are spherical aggregates of tumor cells that are self-organizing and self-renewing. They may originate from single-cell suspensions of tumor cell lines, patient-derived tumor cells, or tumor stem cells cultured in nonadherent substrates.
One assay which makes great use of tumor spheroids is the 3D Tumor Growth Assays (3D TGA), a proven platform for oncology drug development. The 3D TGA provides tumor conditions which more closely represent the human condition than 2D culture.
Within the assay, tumor cells are embedded in a low-stiffness, laminin-rich extracellular matrix. Human mesenchymal stem cells and patient-derived Cancer-Associated Fibroblasts may also be included in the co-culture. This provides the paracrine signaling present in the tumor microenvironment (TME) of solid tumors.
The 3D TGA also has hormones added (e.g. DHT/E2), glucose restricted to ≤7mM, and an acidic pH of 6.8 maintained. This all provides a 3D assay that is “humanized” and TME-aligned for use in profiling PDX-derived cells and drug panels.
Studies have shown a correlation between response to anticancer agents in 3D TGA, in vivo models, and expected clinical outcome.
Tumor Organoids
Organoids are mini 3D versions of organs, grown in vitro. Tumor organoids, specifically, are three-dimensional models of primary tumor tissue obtained from fresh biopsies.
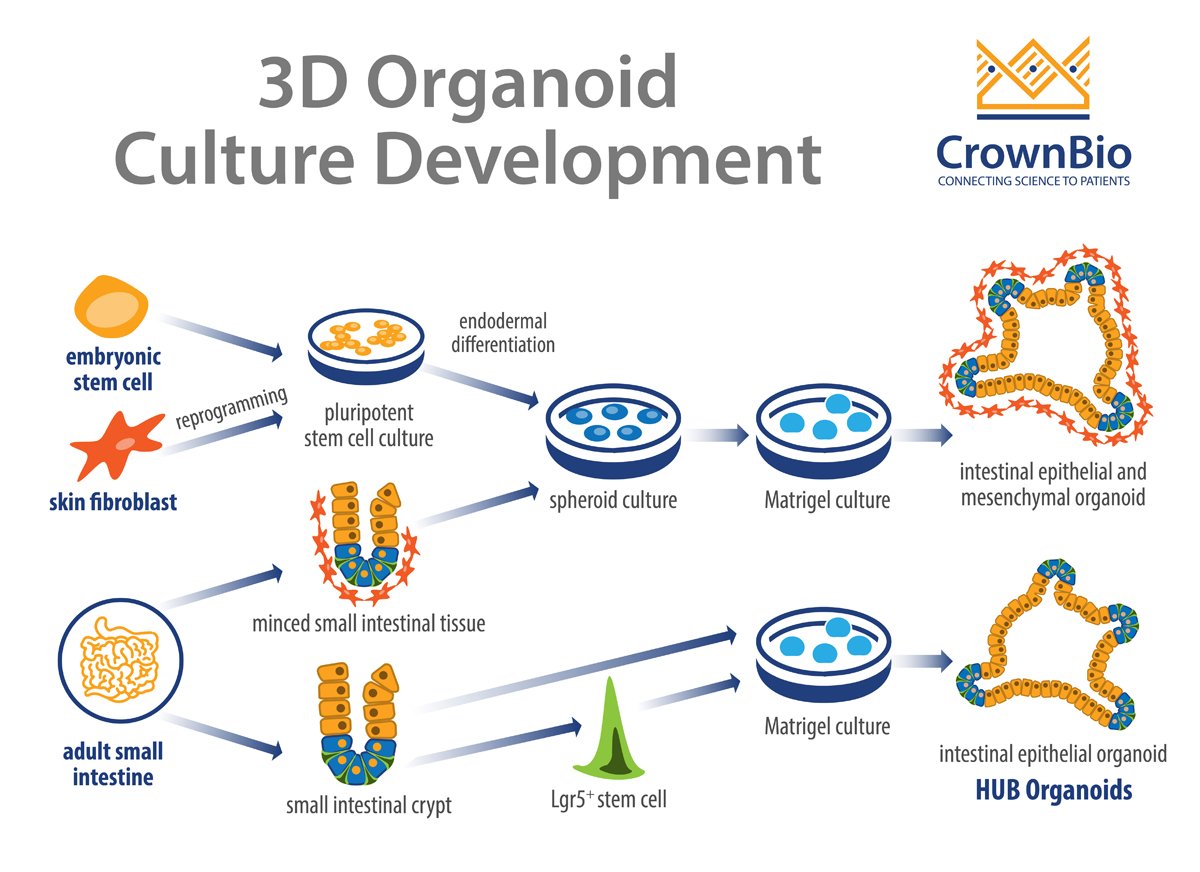
Organoids can be generated from either stem cells or primary tissue (harvested or biopsied). Stem cells can be either embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) whose self-renewal and differentiation properties make it possible to generate organoids containing virtually any cell type.
Organoids are generated by mechanical or enzymatic digestion of the original tumor section into small pieces. Similarly to the 3D spheroid assays, cells are then embedded in a basement membrane matrix such as Matrigel™ or different type of collagen with a cocktail of signaling chemicals to drive the formation of the desired organoid identity.
Alternatively, stem cells can be differentiated into specific cell types, then implanted in a specific 3D environment after maturation.
What is an Organoid?
According to Lancaster and Knoblich, certain criteria need to be fulfilled to fit the definition of an organoid:
- Multiple organ specific cell types: so, organoids must contain more than one cell type of the organ it models.
- Organoids must be able to exhibit some organ specific functions (e.g. excretion, filtration, neural activity, contraction).
- Cells should be organized similarly to the organ.
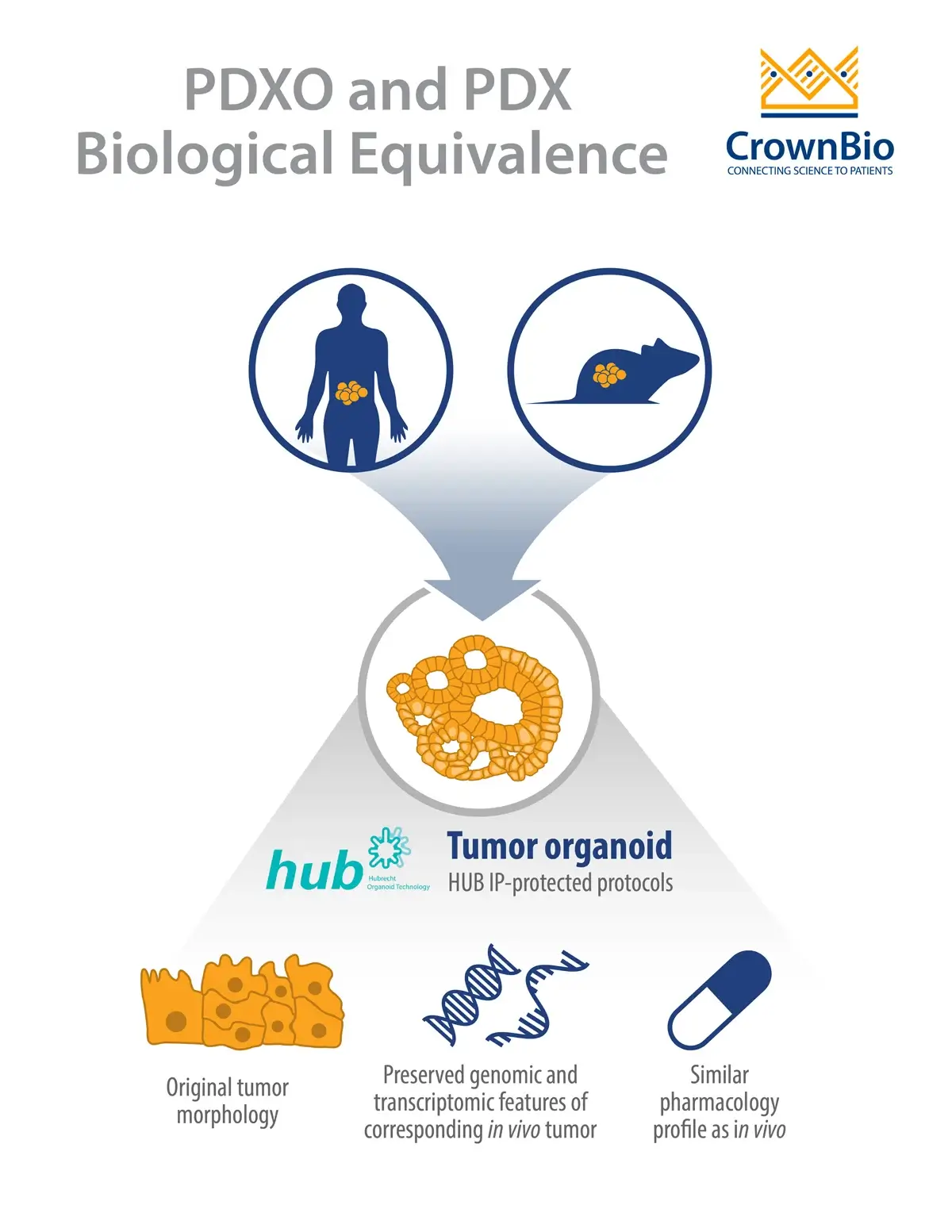
Organoids now represent a growing list of diverse organs. Lung, stomach, pancreas, brain, eye, liver, skin, and many more as well as neoplastic tissues have been generated. Numerous publications show that organoids recapitulate tumor heterogeneity – preserving the molecular features of the primary tumor, including genomic as well as transcriptomic alterations.
A recent study by Sachs et al derived 95 breast cancer organoids from 155 tumors. Gene expression analysis showed that the organoids display representative gene expression profiles of breast cancer subtypes. The organoid morphologies were also retained, and matched the histopathology, and hormone receptor and HER2 status of the original tumor.
This study illustrates the potential of organoid cancer models for drug development and translational medicine.
Organs-on-a-Chip
One of the hot topics in in vitro research is modelling human organs on a chip, physically and chemically mimicking the in vitro environment with microfluidic device technology.
In addition to mimicking the 3D structure of a tissue or organ, organs-on-a-chip more accurately represent physiological conditions and the structural microenvironment, providing more predictive in vitro assays.
Functions of various organs and tissues, such as the lung, liver, kidney, and gut, have been reproduced as in vitro models, and tumor-on-a-chip technology has been extensively published.
Summary
3D in vitro models, such as tumor spheroids and organoids, offer new opportunities for building and applying functional three-dimensional in vitro human tumor translational models for oncology research, immunotherapy studies, and drug screening.