 Humanized mouse models simulate real patient responses for the evaluation of immunotherapeutic drug candidates. This supports more translatable preclinical immuno-oncology research with deeper insight into an agent’s likely performance in clinical trials.
Humanized mouse models simulate real patient responses for the evaluation of immunotherapeutic drug candidates. This supports more translatable preclinical immuno-oncology research with deeper insight into an agent’s likely performance in clinical trials.
Here we review applications for hCD34+ hematopoietic stem cell (HSC)-engrafted humanized models, including recommendations on model selection, study design, timeline, and comments on common misconceptions.
Humanization: hCD34+ vs PBMC
Humanized mouse models are typically generated by one of two approaches:
- Stable engraftment of hCD34+ hematopoietic stem cells from fetal cord blood, leading to a full spectrum of progenitor cell development.
- Transient engraftment with adult peripheral blood mononuclear cells (PBMC) into immunodeficient animals.
The two approaches both have distinct advantages and disadvantages; this post will focus on the generation and use of the more complex hCD34+ HSC humanized models.
Murine Model Selection
There is not one single, perfect humanized model out there for preclinical research. Due to the complexity and variability of the human immune system, you need to select a model appropriate for your study objectives.
Depending on the immune populations of interest and the mechanism of the test article, you can select different models with appropriate immune cell properties. For instance, humanized NSG™ mice (huNSG, from The Jackson Laboratory) or humanized NOG® mice (huNOG, from Taconic) are common baseline transgenic strains for studying T cell response, given their dominating lymphoid lineages.
HuNSG-SGM3 and HuNOG-EXL are similar in their dominant lineages, but also present more abundant functional myeloid lineages.
More advanced models for immuno-oncology research are also now available. The Taconic hIL-6 NOG mouse displays higher macrophage and monocyte populations than the huNOG-EXL mouse, while hIL-15 NOG and hIL-2 NOG mice support NK cell reconstitution. These models are, however, relatively new and less characterized compared to the models above, so further validation and optimization before use may be required.
Selecting Models Based on Lymphoid and Myeloid Reconstitution
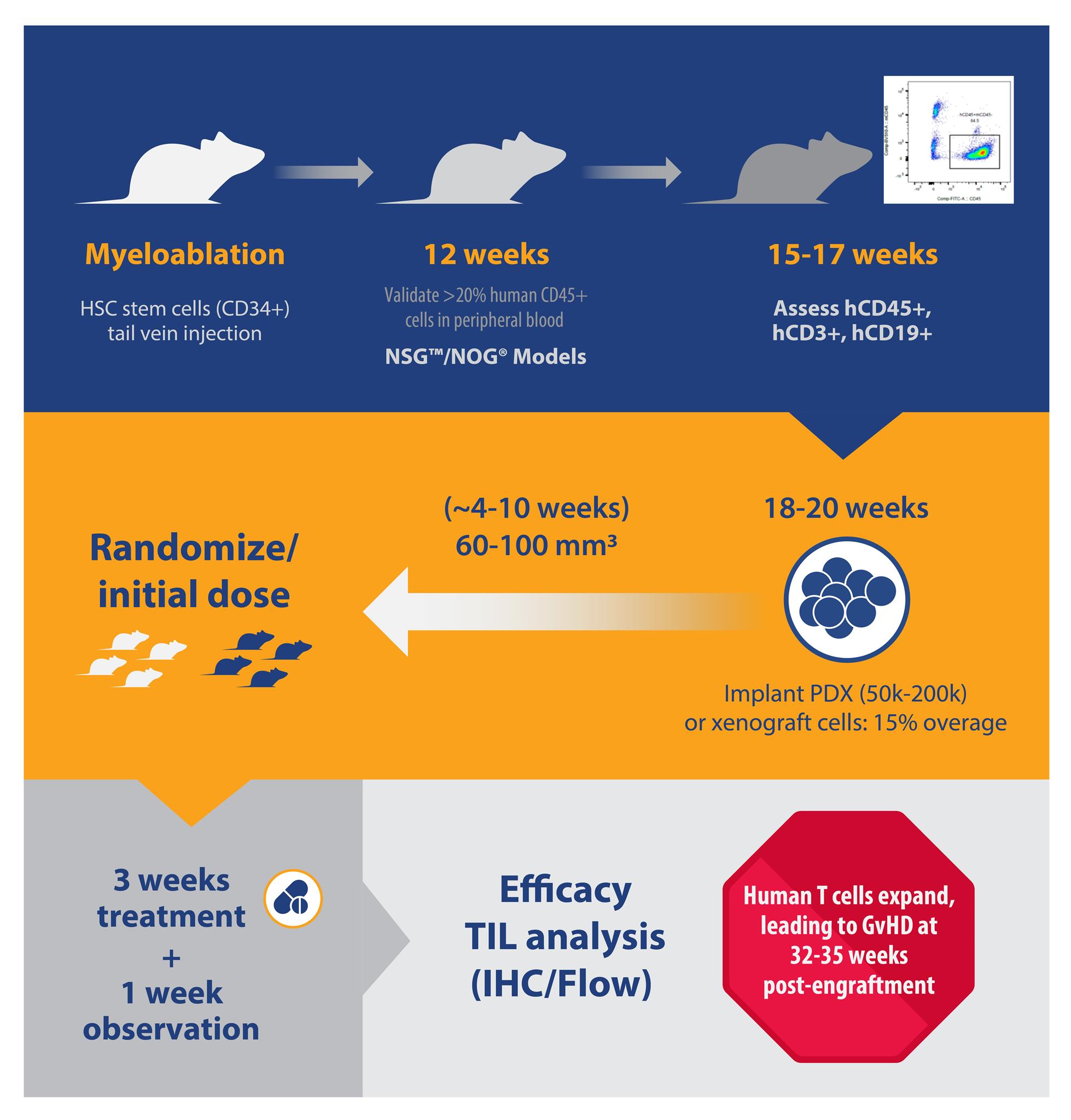
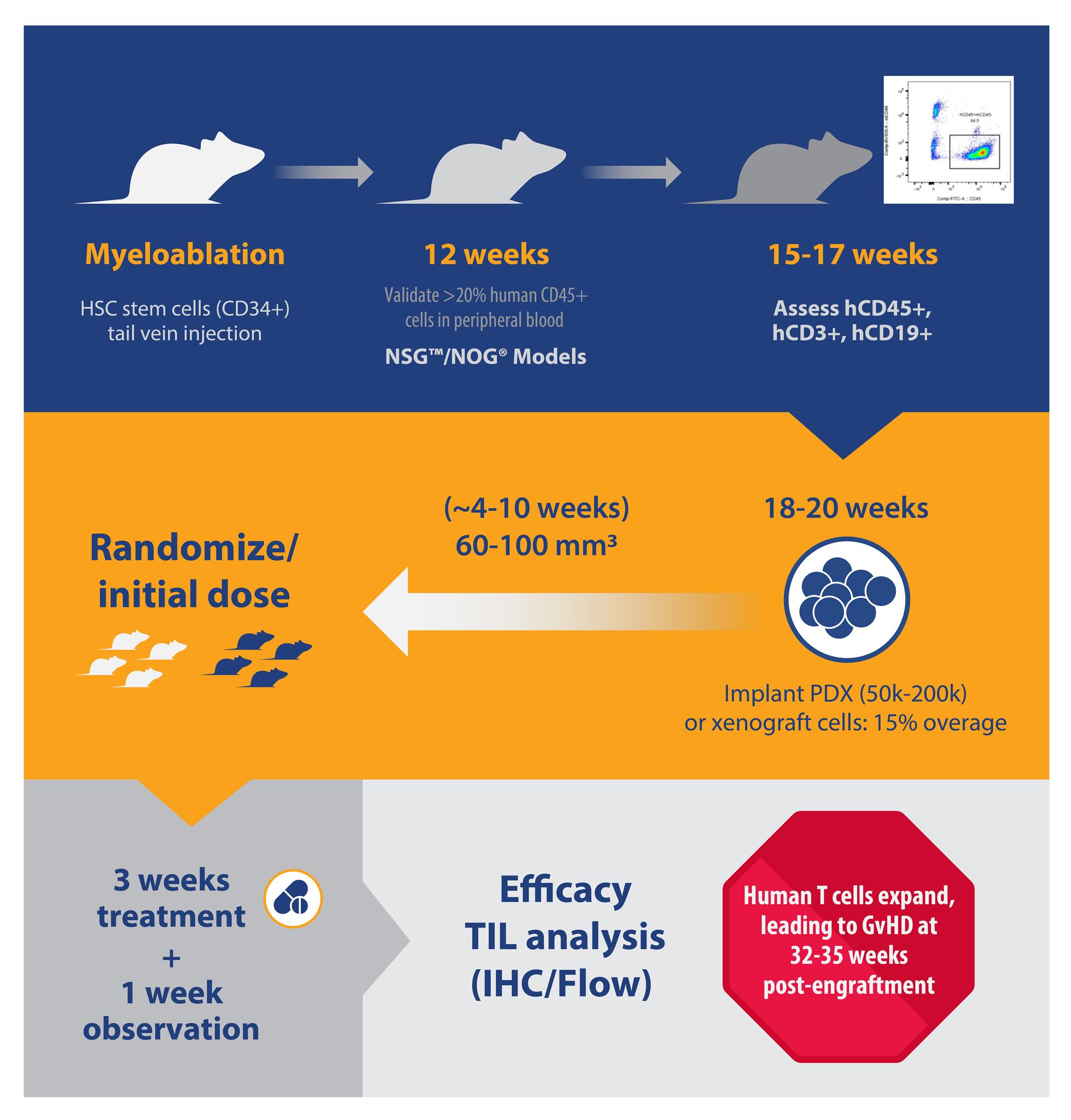
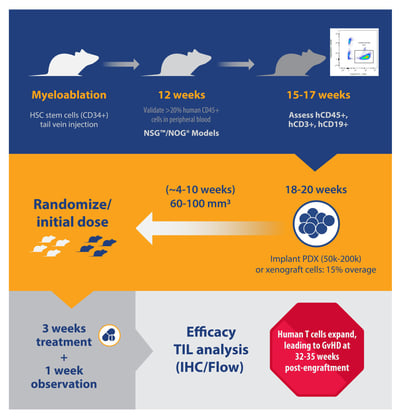
For huNSG and huNOG, the number of donors and animals needed per donor affects the lead time to obtain animals for study. Lead time can vary from two weeks (i.e. for three donors with 15 animals per donor), to months (i.e. more than five donors, 25 animals per donor). This timeline can also be prolonged if specific HLA types are needed.
B and T cell engraftment levels can be detected as early as six and 12 weeks post-engraftment and are typically checked around 15 to 17 weeks post-engraftment for huNSG and huNOG. Percentage population instead of absolute cell counts are typically assessed.
Percent hCD45 varies greatly, between 20-80%, and hCD3+ subpopulations vary between 10-50% for huNSG/huNOG. For huNSG-SGM3/huNOG-EXL, hCD33% ranges between 2 to 25% 15-17 weeks post-engraftment.
Animals with human T or myeloid cell populations closer to the median from a single donor are selected for study. It’s recommended to keep the average mean %hCD45 and %hCD3 similar across all donors in a single study. While this is an attempt to reduce variability, so far no data has demonstrated that variability in %hCD45 and %hCD3 has influenced either tumor growth or drug response.
Animals are typically implanted with tumors at approximately 18 weeks post-engraftment. The engraftment level in these models is typically sustained for up to 35 weeks post-engraftment.
Conventional Xenografts vs Patient-Derived Xenografts?
Traditional, cell line derived xenograft models are inherently less variable, and historically more characterized than patient-derived xenografts (PDX). However, large collections of well-characterized clinically-relevant PDX models offer disease indications which may not be available in conventional xenograft panels.
PDX databases collate model data, such as RNA sequencing, to provide transcription levels for a library of genes. These powerful tools allow identification of specific target antigens to screen for drug candidates and support the quick and easy choice of models of interest.
It’s important to keep in mind that growth validation may not have been done for all PDX models in a collection. In that case, it’s recommended to do a quick model validation before the humanized study, which could potentially add more overall time to a drug development program.
Common Humanized Model Misconceptions
Misconception: Humanized animals can be housed and handled like any immunodeficient animal.
Humanized animals are very fragile and require extra care compared to immunodeficient animals without any engraftment. Animals typically have ruffled, rough coats and may sometimes be hunched.
Specifically, huNSG-SGM3 and huNOG-EXL are much more fragile than huNSG or huNOG; therefore, it’s recommended to implant more mice prior to randomization (i.e. a higher overage). Typically, a 15% overage for each donor is recommended.
Misconception: Tumors do not spontaneously regress in humanized animals.
Occasionally, spontaneous tumor regression occurs in humanized models, probably due to graft versus tumor effect (GvT) where donor T cells attack the grafted PDX cells. The effect can be tumor-dependent, since animals engrafted with the same donor may vary in regression occurring.
This effect is probably not down to HLA-matching, as donor-to-PDX HLA-mismatching doesn’t show an impact on PDX growth or take rate. This is why having a sufficient amount of overage is important, especially if GvT has been seen previously in the same model you’re interested in.
Misconception: Human anti-PD1 or anti-PD-L1 serve as positive controls.
As the leading immunotherapies in the clinic, anti-PD-1 agents such as pembrolizumab (Keytruda®, Merck) or nivolumab, (Opidvo®, Brisol-Myer Squibbs) and anti-PD-L1 atezolizumab (Tecentriq® Roche/Genentech) are desirable drugs to combine with test articles for combination therapy.
However, you should not expect anti-PD-1/anti-PD-L1 to function as a positive control.
The efficacy of these drugs is highly dependent on donor-to-donor variability. Tumors that are PD-L1 positive are not guaranteed to respond to anti-PD-1 treatments.
A recent study compiled eight randomized controlled clinical trials in over 4,000 patients with five types of advanced or metastatic solid tumors. The results showed either anti-PD-1 or anti-PD-L1 monotherapy led to increased survival in patients that were PD-1 positive (34%) and PD-L1 negative (20%) compared to conventional therapies.
Other studies have also shown patients with low or no PD-L1 expression respond to anti-PD-1 therapy. This clearly shows anti-PD-1/anti-PD-L1 do not have a 100% response rate, and that while PD-L1 positive tumors are better responders, this alone is not an adequate biomarker in selecting patients for anti-PD-1/PD-L1 therapy.
Emerging studies have shown higher tumor mutational burden is correlated with greater response for anti-PD-1/PD-L1 therapies, and may be a good starting point to select models.
Misconception: Tumor growth inhibition is the only readout to assess test article efficacy.
Tumor microenvironment and tumor infiltrating lymphocytes (TIL) are important benchmarks in addition to efficacy, measured by tumor growth inhibition (TGI). It is worthwhile to anticipate multiple readouts for a more comprehensive evaluation on the pharmacodynamic properties of test articles.
Immune cell population and distribution can be examined by flow cytometry, immunohistochemistry, and cytokine production by flow, ELISA, or ELISPOT for more robust evaluation of drug properties. Baseline immunophenotypes for PDX tumor-bearing huNSG are often supplied using a 14-color TIL panel.
Summary
hCD34+ HSC humanized models are a useful tool in immunotherapy drug development. Through careful model selection and study design, they can provide a wealth of data on novel human agent efficacy and PD effects to progress immuno-oncology research programs.