Where Do Organoids Fit in Preclinical Cancer Modeling?
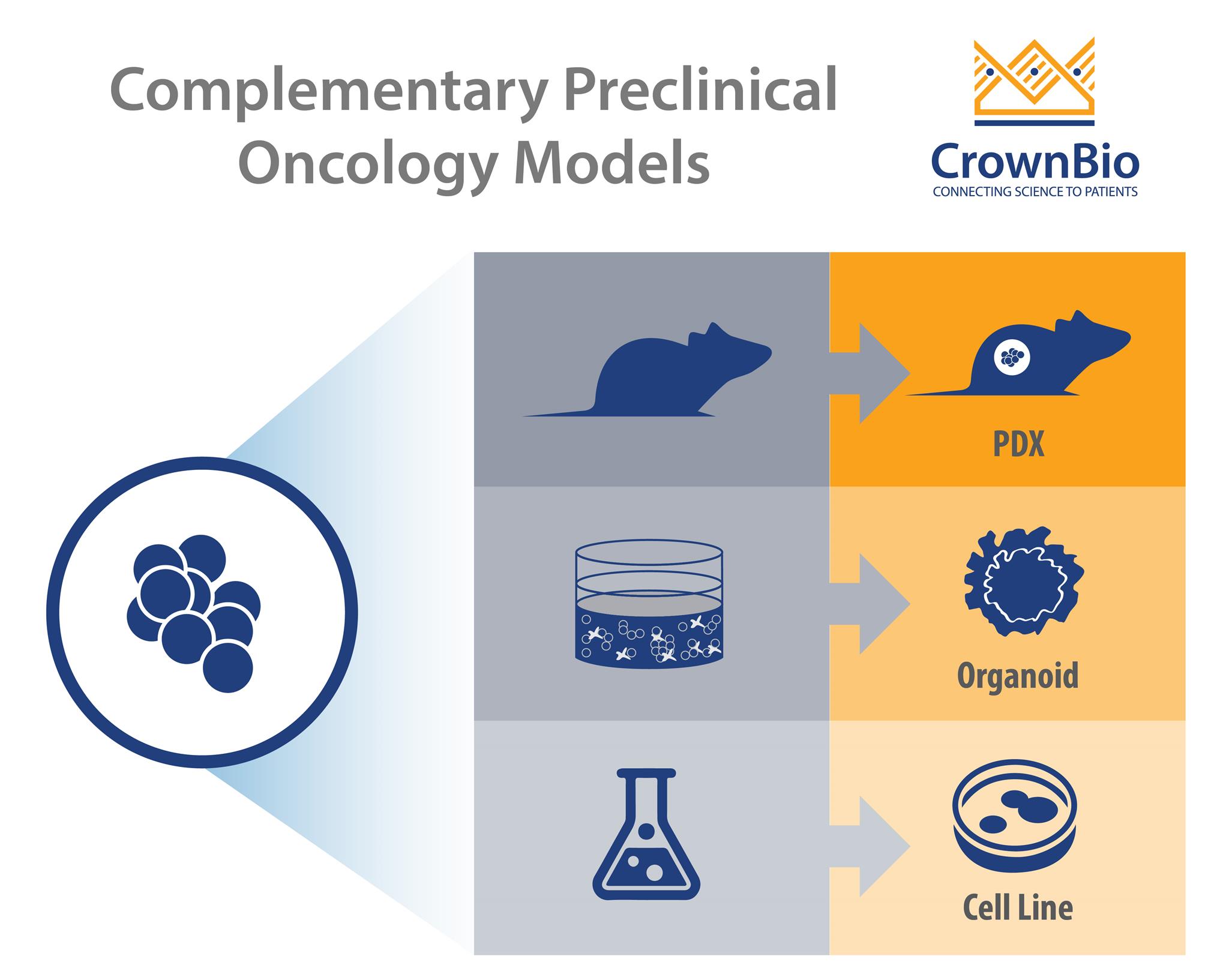
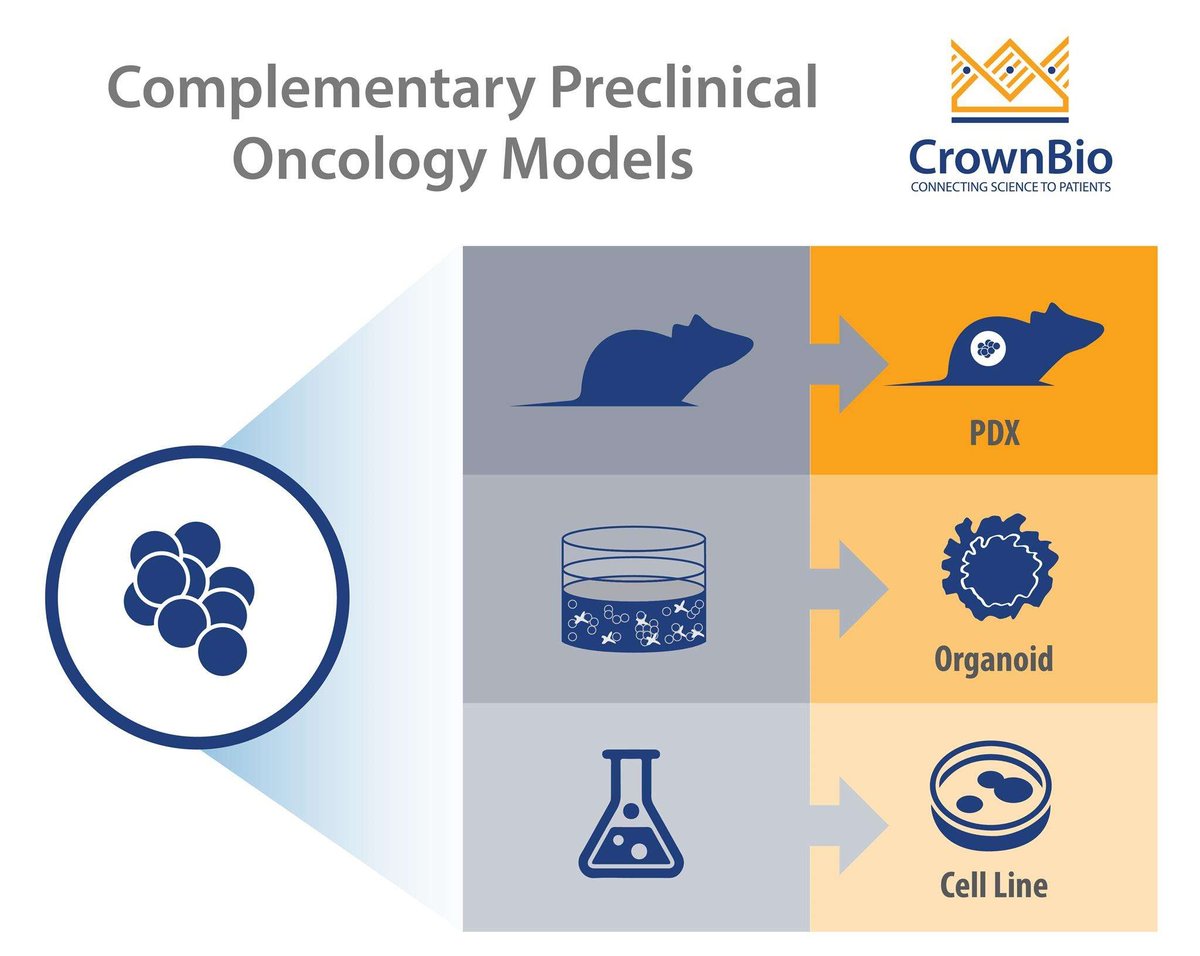
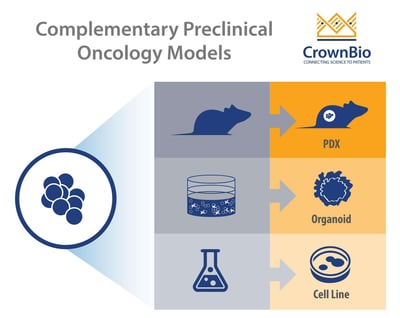 There’s a wide range of new preclinical cancer models being developed, each with their own strengths and best uses. This post looks at 3D cancer organoids and how they fill the gap between simple 2D in vitro assays and in vivo cell line and patient-derived xenograft models.
There’s a wide range of new preclinical cancer models being developed, each with their own strengths and best uses. This post looks at 3D cancer organoids and how they fill the gap between simple 2D in vitro assays and in vivo cell line and patient-derived xenograft models.
Integrating 3D Oncology Models with Traditional Preclinical Resources
The inability of 2D in vitro assays to accurately predict in vivo response is one of the contributing factors to the high attrition rate of cancer therapeutics. Therefore, selecting appropriate preclinical models based on similarity to human biology and disease genotype and phenotype has traditionally relied on animal models.
We have recently discussed the new opportunities that 3D in vitro models, such as tumor spheroids and organoids, can offer. This includes building and applying functional three-dimensional in vitro human tumor translational models for oncology research, immunotherapy studies, and drug screening.
With the variety of oncology cell lines and preclinical models available, it’s now essential to know when to use the right model for the right study role. This includes integrating new models such as 3D cancer organoids into existing preclinical strategies, to fill the gaps between 2D in vitro and conventional in vivo assays.
Cancer Cell Lines for Initial Drug Screening
2D in vitro screening is a mainstay of early preclinical development. Cancer cell lines are usually inexpensive, easy to grow, and readily amenable to high-throughout drug screens of vast libraries of small molecule compounds across large cell line panels.
This can provide information about cell-killing potential, while identifying drug targets and potential biomarkers predicting response. The deep understanding we have of the molecular profiles of a large array of cancer cell lines can facilitate their directed application in drug discovery.
However, it is well known that cell lines adapt to growing on plastic and drift from original disease, losing true disease representation and potential predictive ability. 2D cultures also don’t capture the reality of the 3D tumor biology in situ.
Cell Line Derived Xenografts for Early Stage In Vivo Pharmacology
Transplanting cancer cell lines into immunodeficient mouse models generates conventional, cell line derived xenografts which re-establish a more physiological environment. These models provide a good tool for early stage in vivo drug discovery.
Conventional xenografts carry information learned during in vitro screening forward into the next dimension of drug discovery – in vivo pharmacology. In this case, activity discovered in vitro can be assessed in vivo, in the context of host-determined factors such as ADME and pharmacokinetics.
Xenografts can be run quickly, producing results to compare with a wealth of historic data. This can provide a rapid proof-of-concept as to whether your new agent works in a specific indication, or against a specific overexpressed protein. Typical uses for traditional xenografts include:
- Initial evaluation of multiple potential candidates or a compound series.
- To evaluate and rank efficacy of a new agent against a known cell line target.
- To understand aspects of new drug properties or MOA.
Conventional Xenografts Duplicate Cell Culture Issues
As conventional xenografts are generated from cultured cell lines means they suffer from many of the limitations of 2D cell culture. With xenografts, this includes issues with gene expression and protein function influenced by cell stress combined with clonal selection.
While some publications have reported that cell line xenograft tumors share histological characteristics with their corresponding primary tumors, others have found that these tumors fail to recapitulate primary tumor histology. Moreover, depending on the drug target and/or indication, activity of therapeutic agents on traditional xenograft models may have poor correlation with activity in human patients.
For example, low correlation is observed in studies using pancreatic ductal adenocarcinoma (PDAC) xenografts, probably due to the dense desmoplastic stroma and low blood vessel density found in human PDAC, which are not recapitulated in xenografts of this malignancy.
In this case, genetically engineered mouse models (GEMM) of PDAC offer an alternative model. PDAC GEMM demonstrate similar features to human PDAC, characterized by a dense stroma, which is responsible for a high interstitial pressure and collapsed vessels.
Patient-Derived Xenografts (PDX) For More Predictive In Vivo Assays
PDX models, created by directly implanting patient tumor tissue into immunodeficient mice, removes the issue of in vitro selection pressure. The PDX is then established by serial passage into further animals (P1, Pn, etc.). These models are constantly maintained in vivo, with heterogeneous cell types and a tumor microenvironment closely mimicking that of human tumors.
In this respect, PDX closely recapitulate the genotype and phenotype of patient tumors at establishment. Furthermore, intra-tumor clonal architecture is largely conserved in PDX after serial passaging.
PDX are the ideal tools to use when drug development programs are gearing up for clinical trials, as they have a high degree of translatability - where response to standard of care correlates well with patient clinical response. This provides highly predictive data for guidance on indication or patient clinical stratification and comprehension of resistance mechanisms.
PDX Models Not Instantly Amenable to High Throughput Assays
However, the utilization of the PDX platform is less compatible with large and rapid screening, due to cost and the time needed to establish the PDX tumors in mice. One way to overcome this problem, and to have an integrated drug development program, is to back translate PDX to in vitro and early stage in vivo resources.
Positioned in this respect, cell lines have been derived from PDX maintaining essential histopathological features and genetic profiles of the original patient tumors. This means that any rare fusion or mutation you were targeting in the PDX will still be present for cell screening. This also includes features such as biochemical signaling and response to tumor cell autonomously targeted therapeutics.
The PDX-derived cell lines can also be established as "conventional" xenograft models, again providing the genetic feature of interest in a robust system for early stage drug discovery. PDX-derived cells are also amenable to 3D culture, more closely representing tumor biology in situ. However, establishing these cell lines is also time consuming and is not successful in every case.
Organoids for High Throughput, 3D Large Scale Drug Screening
As we described in more detail in our previous blog, organoids are three-dimensional models of primary tissue obtained from fresh biopsies. Organoids contain stem cells whose self-renewal and differentiation properties make it possible to generate 3D structures containing virtually any cell type.
These cells exhibit spatial organization similar to the corresponding organ and are capable of recapitulating some functions of that organ, providing a highly physiologically relevant system. Moreover, organoids retain cell–cell and cell–matrix interactions that more closely resemble those of the original tumor. Organoids from normal tissues can also be used to characterize any off-target toxicity.
Compared to traditional xenograft models, organoids have higher structural and physiological similarities compared to the patient tumor. This includes a stable genomic profile over extended periods of culture for organoids, and stable drug sensitivity profiles even after prolonged periods in culture.
Compared to PDX models, organoids are established more rapidly and with higher success rate, within a timeframe of 2-3 months between propagation and drug efficacy results. They are accessible for genetic modifications and compatible with large-scale drug screening. Similar drug response patterns are seen for both organoids and PDX models.
PDX models can also be successfully established from tumor organoids, with the matched organoid and PDX showing similar histopathology to the parental tumors from which they were derived.
While PDX models are established and propagated in immunodeficient mice, organoids can also be studied with an immune system component and cocultured with autologous immune cells from the patient to evaluate immunotherapeutics.
Summary
In conclusion, organoids derived from primary tissues fill the gap in the arsenal of preclinical models between 2D cell culture/xenograft and PDX models. As we move forward, organoids are likely to become part of standard preclinical drug development process.
Cite this Article
Bourré, L., (2018) Where Do Organoids Fit in Preclinical Cancer Modeling? - Crown Bioscience. https://blog.crownbio.com/organoids-drug-screening
Related Posts


Navigating Phase 3: Translational & Clinical Support in Drug Discovery
The final stage of drug discovery focuses on translational and clinical support, turning promising preclinical findings into successful human trials.…
Read more
Navigating Phase 2: Preclinical Development and Optimization in Drug Discovery
Phase 2, of the drug discovery process, or preclinical development and optimization, is a critical stage in drug discovery. It bridges early lead…
Read more