Following our post covering the Tumor Necrosis Factor Superfamily of Ligands and Receptors, we’re going to take a closer look at specific family members important in immuno-oncology. First up is the OX40 receptor and its binding partner, OX40-Ligand (OX40L).
Following our post covering the Tumor Necrosis Factor Superfamily of Ligands and Receptors, we’re going to take a closer look at specific family members important in immuno-oncology. First up is the OX40 receptor and its binding partner, OX40-Ligand (OX40L).
Important Targets in T Cell Co-Stimulation
OX40 is now at the forefront of the field, which has been called “T cell co-stimulation”. Immune co-stimulators work by providing the signal that promotes the expansion and proliferation of killer CD8 and helper CD4 T cells.
While immune checkpoint therapies are “releasing the brakes,” co-stimulators are “stepping on the gas.” Both mechanisms are required for a robust immune system response.
Receptor and Ligand Characteristics of the TNFR Superfamily
OX40 (also known as CD134, ACT35, and TNFRSF4) is a 50kD type 1 transmembrane glycoprotein. The extracellular N-terminal portion of OX40 is 191 amino acids, containing three complete and one truncated cysteine-rich domains (CRDs) which are characteristic of the Tumor Necrosis Factor Receptor (TNFR) superfamily. The intracellular region consists of 36 amino acids.
OX40L (also known as gp34, CD252, and TNFSF4) is a 34kD type II transmembrane glycoprotein. It is expressed as a trimer and has a TNF homology domain; therefore, it is structurally similar to other molecules of the TNF superfamily and has some sequence homology.
OX40 and OX40L Genes Clustered with Other Family Members
The gene for OX40 is clustered on human chromosome 1 (mouse chromosome 4) with several other TNFR family molecules, e.g., TNFR2, 4-1BB, HVEM, CD30, GITR, and DR3. The OX40L gene is on human and mouse chromosome 1, clustered with genes for two other TNF family members, FasL and GITRL.
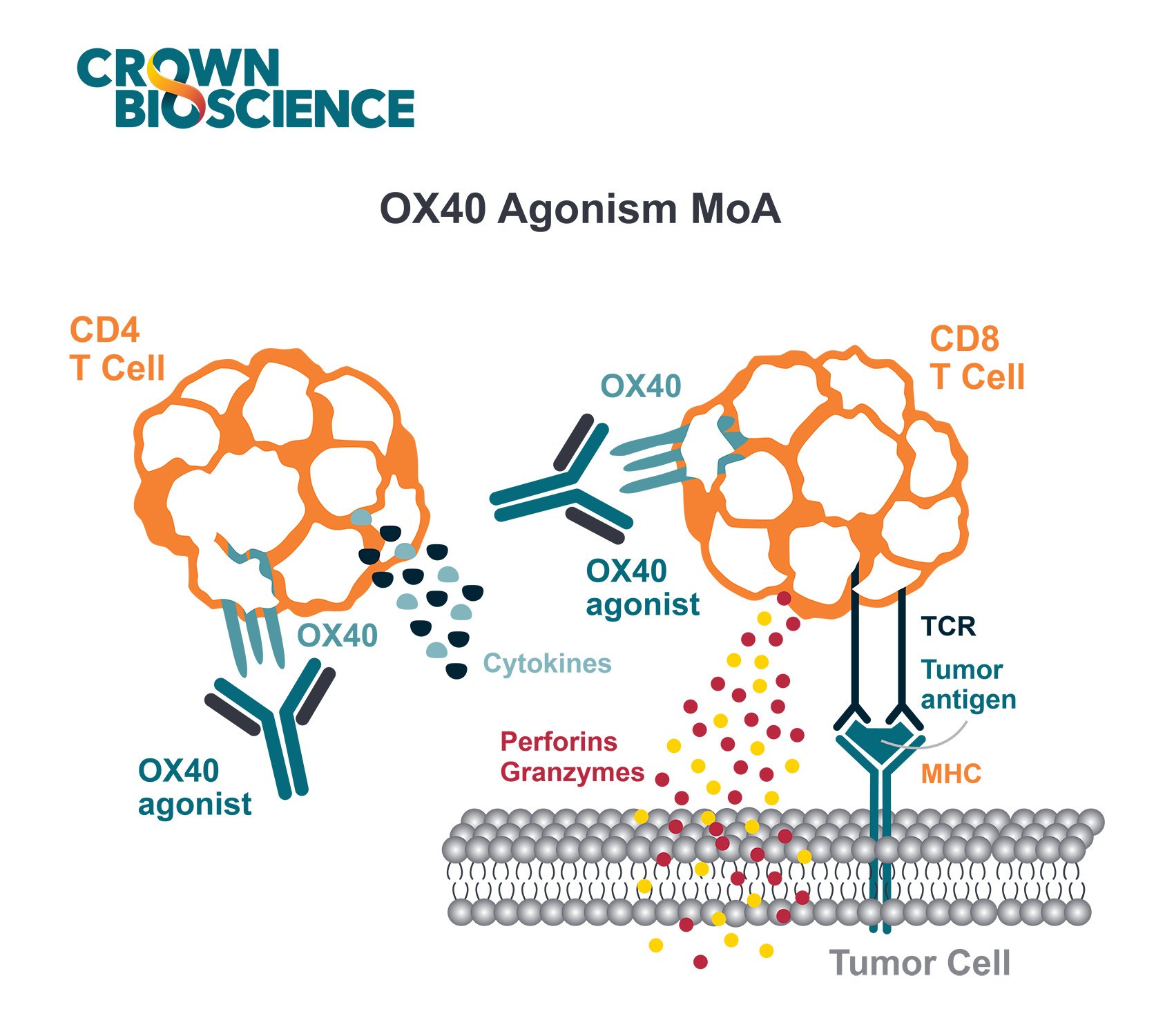
OX40 Ligand Binding Modulates T Cell Activation and T Cell Effector Function
The OX40 receptor is expressed primarily on CD8+ T cells, NK cells, NKT cells, and neutrophils. OX40L is expressed on dendritic cells (DCs), B cells, macrophages, and at sites of inflammation (e.g., activated endothelium).
OX40 is a co-stimulatory receptor not constitutively expressed on resting naïve T cells, instead, it serves as a secondary co-stimulatory immune checkpoint molecule. OX40L is also not expressed on resting antigen presenting cells, but is present following their activation.
Binding of OX40 to its ligand modulates T cell activation and T cell effector function.
The interaction between OX40 and its ligand, OX40L, plays a crucial role in the regulation of T cell responses, particularly in antigen-specific CD4+ T cells. This interaction is pivotal, providing costimulatory signals that not only promote cell proliferation but also enhance effector functions such as cytokine production and improve cell survival. Additionally, the synergy between OX40 and OX40L is instrumental in boosting both effector function and memory recall responses in a spectrum of T cells, including CD4+ and CD8+ subsets.
Moreover, the dynamic involvement of OX40 and OX40L extends to the regulation of T regulatory cells (Tregs). With OX40 being constitutively expressed in mice and inducibly expressed in human Tregs, the activation through this axis can mitigate the suppressive functionalities of Tregs, hinder their development from effector T cells, and lead to a decreased expression of the transcription factor Foxp3.
Furthermore, this interaction between OX40 and OX40L is critical in triggering T helper (Th) cell responses, notably the Th2 response initiated by basophils, which is a significant precursor to allergic airway inflammation. In-depth investigations into the role of basophils in various mouse models of helminth infections have underscored their importance in Th2 cell development, primarily via IL-4 secretion following their transitory recruitment into draining lymph nodes. Despite these insights, studies in basophil-deficient mice have highlighted that basophils are not essential for tumor establishment in the long term. However, their presence within experimental tumors has been associated with promoting a Th2 environment and favoring the polarization of macrophage phenotype 2 (tumor-associated macrophage), as seen in numerous types of tumors.
Targeting OX40 in Preclinical Studies: Improved Tumor Free Survival and Immune Memory Response
Preclinical studies have shown that using anti-OX40 mAbs and OX40L-Fc fusion proteins can increase antitumor immunity and improve tumor free survival. OX40 dependent antitumor immunity required the expansion of CD8 and CD4 T cells, with a proportion of mice showing evidence of a strong memory sufficient to provide resistance upon tumor re-challenge.
Research in preclinical models suggests that drugs aimed at the OX40 receptor can activate T cells within tumors, thereby bolstering anti-tumor immunity and enhancing the immunological memory that contributes to tumor-free longevity. These therapeutic approaches, including anti-OX40 monoclonal antibodies and OX40L-Fc fusion proteins, have been instrumental in the expansion and fortification of both CD4 and CD8 T cell populations. Such advancements have been observed across various tumor types, including sarcoma, breast cancer, colon cancer, and glioma, where the binding of OX40 to OX40L activates tumor-targeting T lymphocytes.
In vivo experiments employing adenovirus vectors for the genetic engineering of tumor cells to express OX40L have showcased anti-tumor effects across different cancer models—including lung cancer, melanoma, and colon cancer. Additionally, the utilization of OX40 agonists, such as OX40L-Fc, in a mouse sarcoma framework has led to a favorable alteration of the tumor microenvironment via the activation and proliferation of effector T cells. Studies by Gough and colleagues have revealed that treatments with OX40 agonists result in a significant uptick in intra-tumoral CD8+ T cells and a simultaneous reduction in regulatory T cells, creating an immune-promoting tumor microenvironment characterized by decreases in transforming growth factor-beta, myeloid-derived suppressor cells, and macrophages. Moreover, OX40L therapies, such as OX40L immunoglobulin conjugates, delivered in murine tumor models, have demonstrated a dose-responsive anti-tumor action.
Piconese et al.'s work indicates that Tregs expressing OX40 can be stripped of their immune-suppressive capabilities following OX40 engagement, with intratumor administration of anti-OX40 monoclonal antibodies leading to total tumor rejection through an adaptive immune response. In related research, activating OX40 in Tregs with an agonist (the OX86 monoclonal antibody) diminished these cells' immunosuppressive function, reducing IL-10 production and bolstering effector memory T cell activity. Findings from Bulliard and associates have shown that OX40 agonists can clear tumor-infiltrating Treg cells in mouse models by stimulating the Fcγ receptor, contributing to anti-tumor effects.
The latest studies have explored combined strategies, such as OX40 stimulation alongside PD-L1 blockade in cytotoxic T cells, to further potentiate anti-tumor responses. These promising outcomes from preclinical research targeting the OX40/OX40L pathway are now setting the stage for clinical trials aimed at assessing the efficacy of novel agents that harness the OX40 co-stimulatory receptor in various cancers, a subject that is further explored in subsequent sections.
Treg Cell Effects – Both Depletion and Expansion Observed
Preclinical studies have also shown that OX40 agonists might exert their anticancer activity by depleting the number of FoxP3+ regulatory T (Treg) cells, which express high levels of OX40.
In mice, OX40 is constitutively expressed on Treg cells, which is in contrast to humans where expression is induced - there is low/absent expression on peripheral Treg cells but higher OX40 expression on human Treg cells isolated from sites of inflammation (e.g., tumors).
However, others have observed Treg cell expansion, suggesting that anti-OX40 can push Treg cells in both directions, depending upon the context of stimulation and the cytokine environment.
Clinical Trials Ongoing Including Combination Therapy
Recently, Phase I monotherapy studies [NCT01644968] have been conducted with an OX40 agonist (9B12, mouse monoclonal anti-OX40 antibody) in patients with metastatic solid tumors. Promising results were observed, showing the strong bioactivity of the compound, although no antitumor responses were seen. 12 out of 30 patients showed regression of at least one metastatic lesion, and the agent was well tolerated with mild to moderate side effects.
In order to increase anti-OX40 effect, a variety of combinatory therapy strategies are being investigated.
Currently, agonistic OX40 monoclonal antibodies (e.g., MOXR0916, PF-04518600, MEDI0562, MEDI6469, and MEDI6383) are being evaluated in several Phase I/II clinical trials either as monotherapy or in combination with other immunomodulating agents:
- Durvalumab (anti-PD-L1 antibody) [NCT02221960]
- Tremelimumab (anti-CLTA-4 antibody)/rituximab (anti-CD20 antibody) [NCT02205333]
- utomilumab (anti-CD137 antibody) [NCT02315066]
- atezolizumab (anti-PD-L1 antibody)/bevacizumab (anti-VEGF-A antibody) [NCT02410512] (8).
OX40 Combination Regimens Expanding Immunotherapy Benefits
The use of OX40 targeted co-stimulatory agonistic antibodies with other anticancer approaches, including immunotherapy, are promising approaches to overcoming the suppressive tumor microenvironment and expanding the cohort of patients that benefit from immune mediated cancer therapies.

Figure. 1 Anti-OX40 in combination with aCTLA-4 or aPD-1/aPD-L1 enhances T cell proliferation, survival, and effector function while decreasing immunosuppression within the tumor microenvironment.
References and Further Reading:
- Linch SN et al. OX40 Agonists and Combination Immunotherapy: Putting the Pedal to the Metal. Front Oncol. 2015;5: 34.
- Jensen SM et al. Signaling through OX40 enhances antitumor immunity. Semin Oncol. 2010;37(5): 524-32.
- Gough MJ et al. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J Immunother. 2010;33(8): 798-809.
- Song A et al. Cooperation between CD4 and CD8 T cells for anti-tumor activity is enhanced by OX40 signals. Eur J Immunol. 2007;37(5): 1224-32.
- Ruby CE et al. Cutting Edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J Immunol. 2009;183(8): 4853-7.
- Xiao X et al. New insights on OX40 in the control of T cell immunity and immune tolerance in vivo. J Immunol. 2012;188(2): 892-901.
- Curti BD et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73(24): 7189-7198.
- Aspeslagh S et al. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2016;52: 50-66.
- Thapa, Bicky, et al. "OX40/OX40 ligand and its role in precision immune oncology." Cancer and Metastasis Reviews (2024): 1-13.
- Yadav, Rashi, and William L. Redmond. "Current clinical trial landscape of OX40 agonists." Current Oncology Reports 24.7 (2022): 951-960.