Tumor Heterogeneity in Preclinical Oncology Models
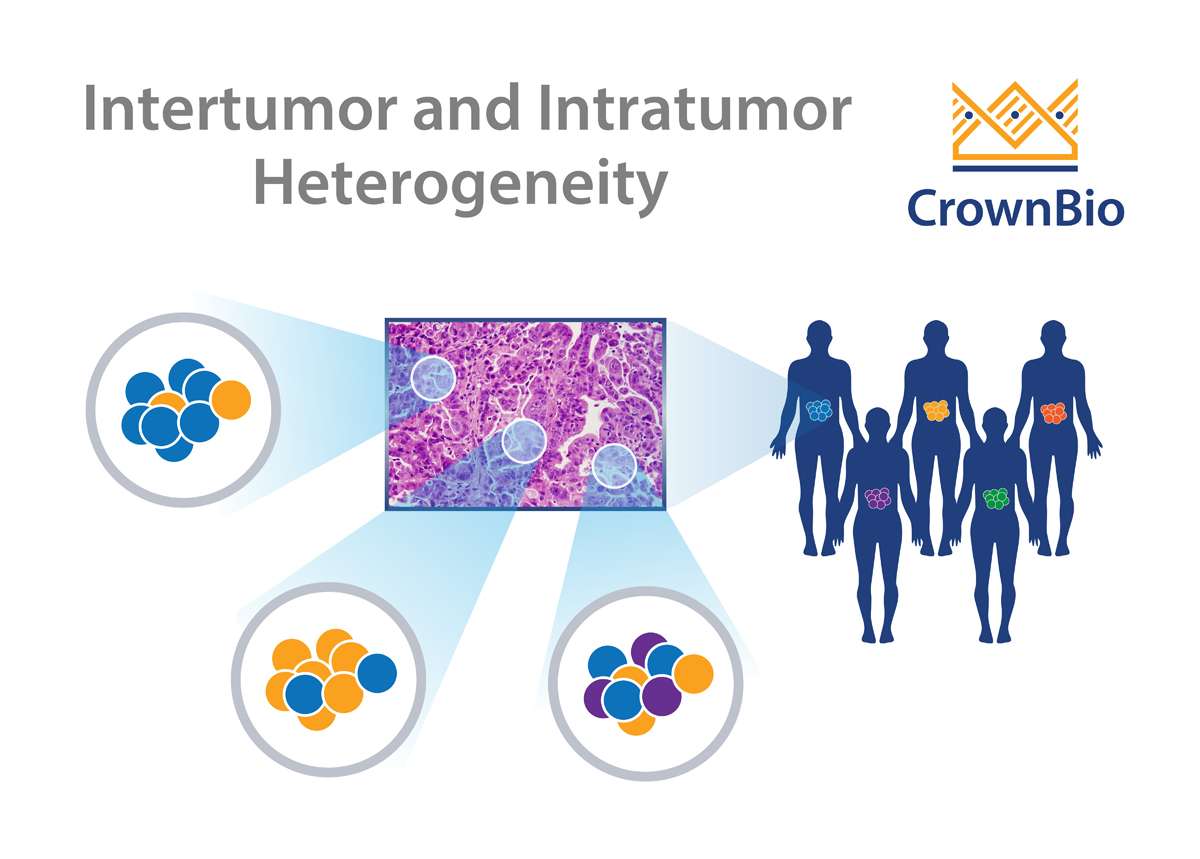
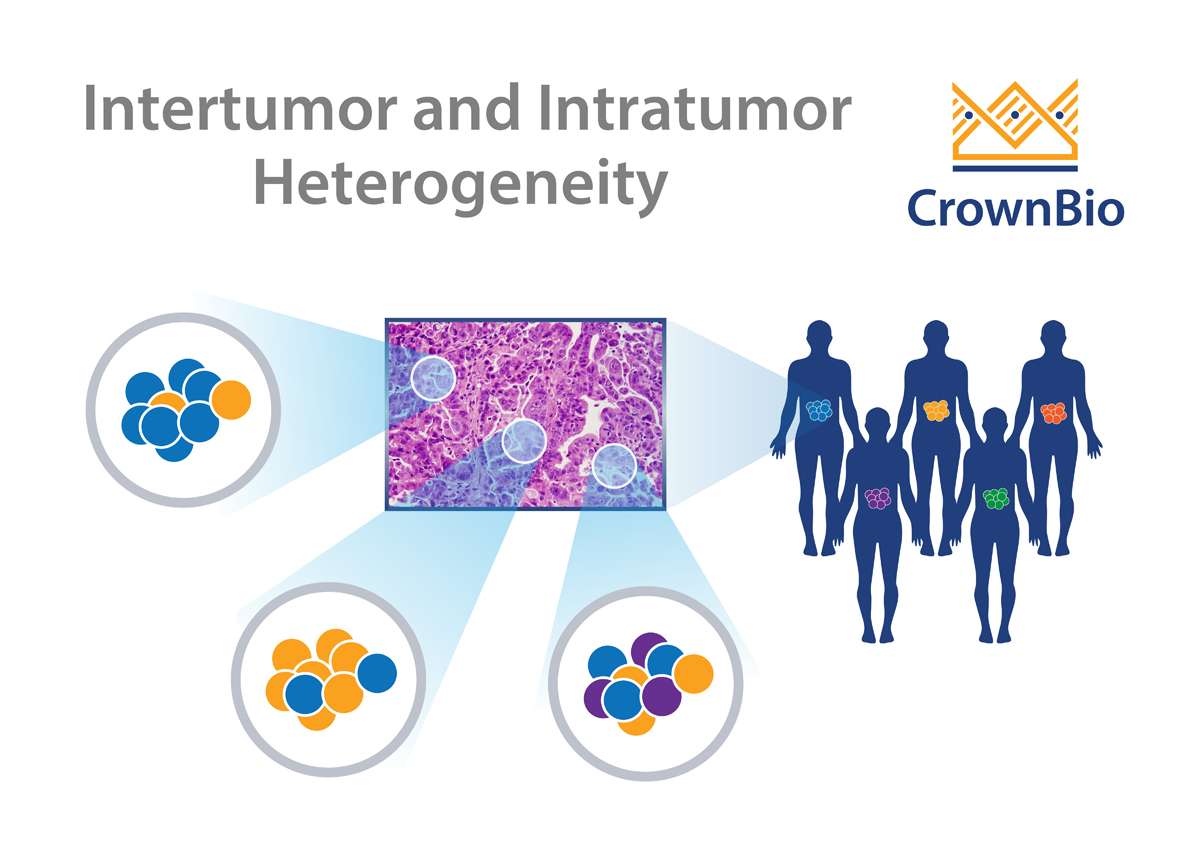
 Real tumors are complex and heterogeneous, which has long frustrated efforts to recapitulate the disease in a controlled setting. What causes tumor heterogeneity, and can it be effectively reproduced in preclinical oncology models?
Real tumors are complex and heterogeneous, which has long frustrated efforts to recapitulate the disease in a controlled setting. What causes tumor heterogeneity, and can it be effectively reproduced in preclinical oncology models?
Tumor Heterogeneity
It was back in the 1970s when tumor progression was first proposed to be an evolutionary process, later determined to be under continuous Darwinian selection.
Generally, tumors are detected after undergoing many rounds of cell division, during which they acquire random, novel somatic mutations. The driver events (a minority subset) may provide the evolutionary advantage which allows cancer cells to grow better than others.
Cancers frequently display substantial intra-tumor heterogeneity in virtually all distinguishable phenotypic features such as metabolism, immunogenicity, and metastasis. The nature of this tumor heterogeneity can have profound implications both for tumor development and therapeutic outcomes.
What Causes Tumor Heterogeneity?
The most common view on the origin of tumor heterogeneity is that it arises from cancer stem cells. These cells have the ability to self-renew and evolve different phenotypic cell populations with strong tumorigenicity.
Intra-tumor heterogeneity is thought to de derived from a number of factors, including genome, transcriptome, proteome, and stromal heterogeneity. This results in tumors which show different levels of sensitivity or resistance to treatment.
Recently, a variety of new technologies helped assess the clonal content of tumors. This includes multi-regional and single cell next generation sequencing, as well as research autopsies.
Changes in clonal content over time, and during treatment, can now be easily tracked. This provides novel insights into cancer evolution and the mechanisms underlying treatment resistance.
Tumor Heterogeneity in Cell Lines and Traditional Xenografts
Preclinical cancer models play a crucial role in oncology drug discovery. Over the years, “traditional” cell line derived xenograft models have played a key part in the process.
Collections such as the NCI-60 cancer cell line panel have been used for anticancer drug screening for over 25 years. The panel has significant limitations, however, such as a lack of prognosis in drug efficacy studies. Despite being a tremendous step forward in drug discovery research, these models still fail to mimic the enormous complexity of human cancers, including tumor heterogeneity and tissue architecture.
Moreover, it’s not accurately known how conventional xenograft models differ from their source tissue genomically, transcriptomically, and proteomically. Traditional xenografts also lack clinical information such as therapeutic response, stage of disease, treatments administered, etc.
More Clinically Relevant Tumor Heterogeneity in PDX Models
The need for improved models lead to the discovery of patient-derived xenografts (PDX). These models are generated by directly engrafting fresh human tumor tissue into immunodeficient mice.
PDX models mostly retain the principal histological and genetic characteristics of their donor tumor. These models have been shown to be predictive of clinical outcomes and are being used for preclinical drug evaluation, biomarker identification, biological studies, and personalized medicine strategies.
The demand for these models has grown exponentially in recent years as they closely mimic patient tumors with regard to tumor microenvironment and heterogeneity. PDX also exhibit a high degree of genomic stability compared to the parental tumors.
Conclusion
In conclusion, PDX models continue to be a preferred model in biomarker development, drug discovery, and evaluation. They capture more of the inter-patient heterogeneity seen across patients, providing a more patient-relevant preclinical model.
Cite this Article
Chakravarthi Venkata Srinivasa, PhD, (2019) Tumor Heterogeneity in Preclinical Oncology Models - Crown Bioscience. https://blog.crownbio.com/pdx-tumor-heterogeneity