Acute myeloid leukemia (AML) is the most common form of aggressive hematologic malignancy affecting adults. AML is characterized by the rapid growth of abnormal white blood cells that accumulate in the bone marrow and interfere with the production of normal blood cells. AML is a relatively rare disease, however its incidence increases with age, thus it is expected to become more prominent as the global population ages.
The FLT3 mutations in AML were identified 15 or more years ago. They consist in an internal tandem duplication (ITD) of the FLT3 gene, resulting in its constitutive activation. FLT3 duplication occurs in approximately 25% of patients with AML and is associated with poor prognosis.
FLT3 encodes for a cytokine receptor with an intrinsic tyrosine kinase activity. It is important for B and T cells development and it auto-activates by clustering on the surface of blood cells progenitors to trigger blood cell survival, proliferation and differentiation.
AML patients with FLT3-ITD have very aggressive disease, which is difficult to treat. The traditional therapeutic approach for these patients included intensive induction chemotherapy to reduce the number of leukemic cells to an undetectable level (remission), followed by consolidative chemotherapy to eliminate any residual undetectable disease and achieve a cure. Patients typically can get into remission, but they very often relapse and die shortly thereafter. Moreover because of its toxic effects on the immune system, induction therapy may not be offered to the very elderly, and the options may include less intense chemotherapy or palliative care. For patients with relapsed AML hematopoietic cell transplantation (HCT) remains the only option.
With the development of more specific and potent small molecules or tyrosine kinase inhibitors (TKIs) the receptor tyrosine kinase activity of FLT3 became a popular target in the attempt to obtain better, more durable responses.
Multiple small molecule inhibitors of the FLT3 tyrosine kinase have been studied preclinically and in clinical trials. Nevertheless, questions remain regarding the optimal timing and schedule for incorporation of FLT3 inhibitors into treatment regimens. Multiple trials, including those with sorafenib, lestaurtinib, and midostaurin, studied the combination of FLT3 inhibitors with standard chemotherapy. Nevertheless the optimal combination of FLT3 inhibitors with cytotoxic agents, leading to improvement in overall survival has yet to be clearly identified.
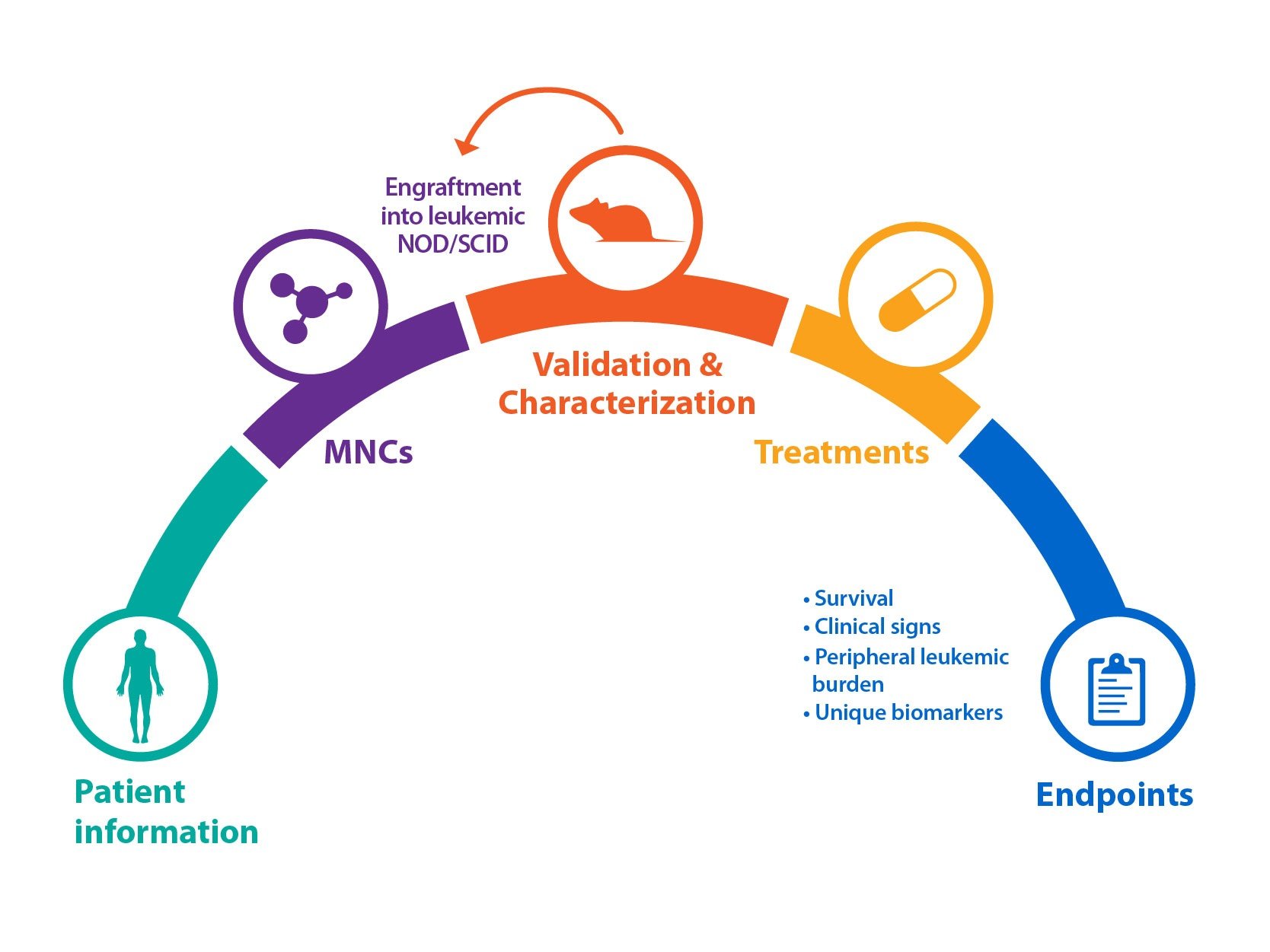
Crown Bioscience has a longstanding interest in modeling blood cancer diseases. At Crown we have developed FLT3-ITD AML patient-derived blood cancer models by serial transplantation of primary patient cancer tissues. All our blood cancer models (HuKemia®) show similar disease manifestation to human patients providing a unique opportunity for companies interested in target discovery, target validation, and evaluating drug response in a clinically relevant model. In addition to our AML resources, Crown’s HuKemia include acute lymphocytic leukemia (ALL) and Non-hodgkin’s lymphoma (NHL) models. Crown DLBCL-NHL model with MyD88 and CD79B mutation was recently showcased during the 57th American Society of Hematology (ASH) Meeting.
To complement and enrich our HuKemia collection we have validated a comprehensive collection of cell line-derived xenograft (CDXs) models among which the subcutaneous MV4-11 model demonstrates clear sensitivity to FTL3 inhibitors.
For more information on our blood disease models and services, contact us today.
DISCLOSURE STATEMENT
Crown Bioscience provides preclinical models and services for translational oncology and is not qualified to provide medical advice. For more information on clinical trials recruitment and current treatment options please refer to your doctor or visit the National Cancer Institute (NIH) and the National Health Service (NHS) websites.