 Learn more about T cell receptors in personalized T cell therapy, including TCR diversity, repertoire, and how TCR are profiled and analyzed -- including new MHC-tetramer methods.
Learn more about T cell receptors in personalized T cell therapy, including TCR diversity, repertoire, and how TCR are profiled and analyzed -- including new MHC-tetramer methods.
Adoptive T Cell Therapy and TCRs
T cell receptors (TCR) play an important role in adoptive T cell therapy (ACT), an effective new treatment approach which induces durable regression of blood cancers. The TCR recognizes malignant cells and presents them for killing by cytotoxic T lymphocytes (CTL).
This can be accomplished by using natural T cells to specifically recognize and eliminate widespread target cells, or autologous T cells can be modified to express specific TCR with high affinity against either tumor associated antigens (TAA) or tumor specific antigens (TSA) presented by a tumor cell’s major histocompatibility complex (MHC). Targeting selected antigens within a patient’s tumor cell repertoire, allows for highly personalized cancer therapy.
This post looks specifically at the role of the TCR within ACT, considering the importance of the T cell repertoire and how to perform T cell receptor profiling.
T Cell Receptor Diversity
Our immune system is equipped with over 300 billion T cells with more than 300 million TCR specificities. T cell function is dependent on TCR signaling. Engagement of a TCR with a specific antigen stimulates downstream signaling cascades. These cascades result in T cell proliferation, activation, and cytokine production such as IFNγ, IL-2, and granzyme B, all of which are critical for killing tumors expressing that specific antigen.
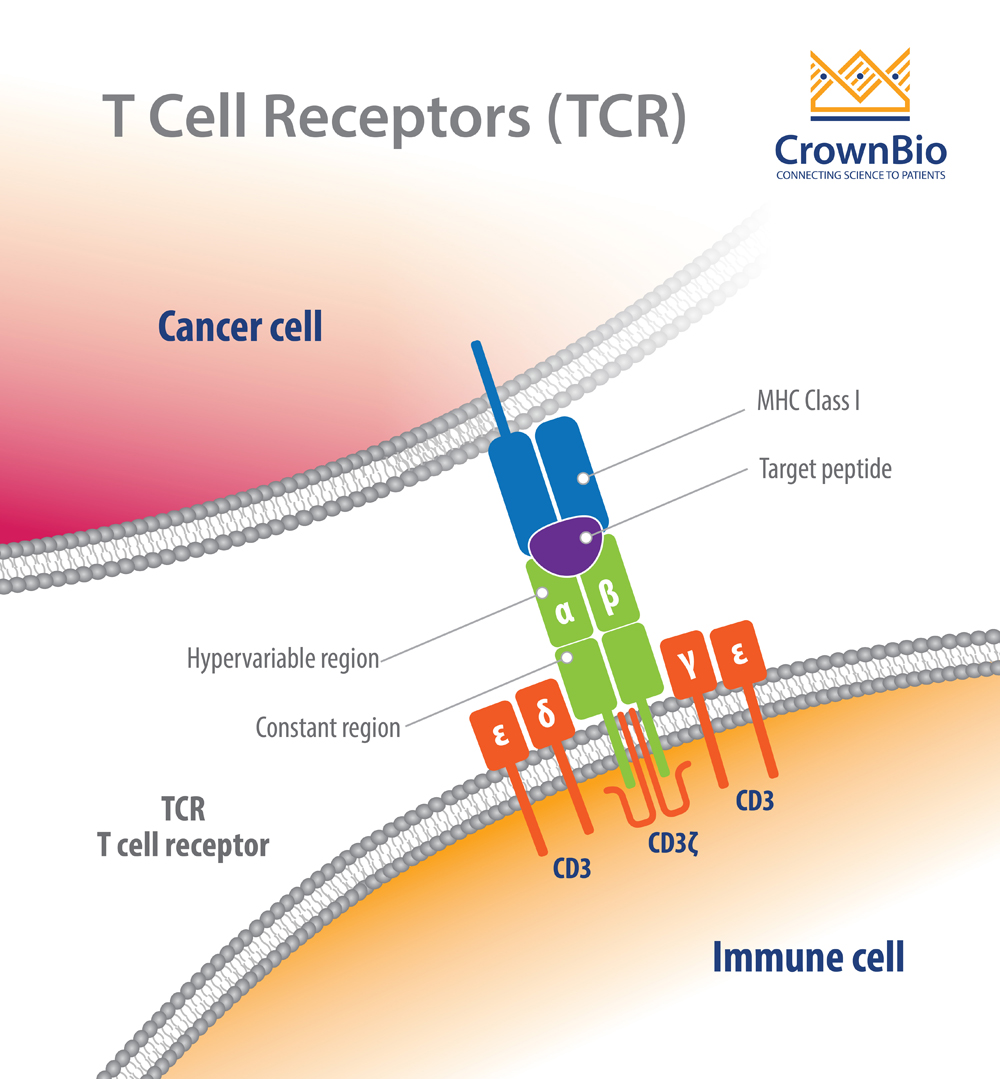
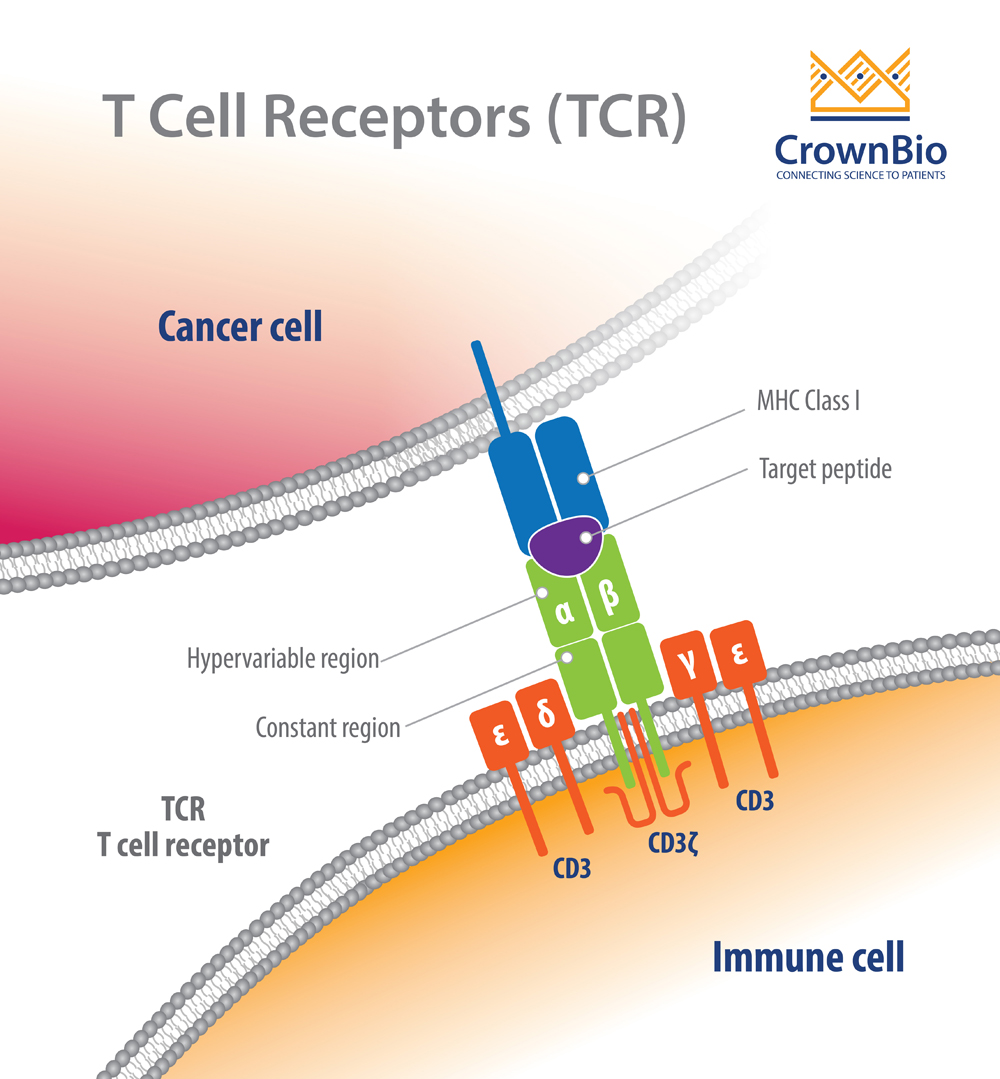
TCRs are highly diverse heterodimers, the majority of which are comprised of a combination of α and β chains or of γ and δ chains. Each TCR is individual, possessing a unique antigen specificity determined by the antigen binding site structure.
This structure arises from V(D)J recombination - the variable region of TCRα and δ chains is encoded by a number of variable (V) and joining (J) genes, and TCRβ and γ chains are additionally encoded by diversity (D) genes.
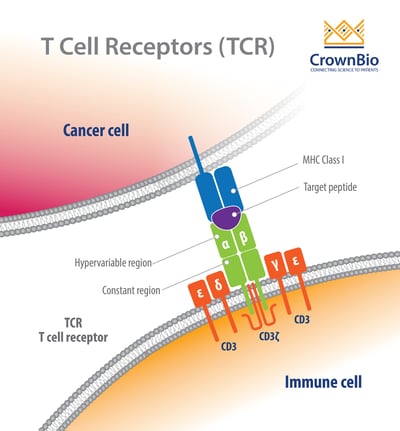
For each final recombined TCR, the α and β chains bind to the peptide MHC (pMHC) on antigen-presenting cells (APC).
The Importance of the TCR Repertoire in Effective Tumor Killing
The TCR repertoire is defined as the total TCR expressed within the T cell population of an individual. The repertoire is dynamic and changes significantly with the onset and progression of disease including cancer, autoimmunity, inflammation, and infection.
In the context of cancer, CTL effectively kill tumor cells upon recognition of TAA and TSA. By analysing tumor infiltrating lymphocyte repertoires, specific and effective clonal types involved within cell killing can be identified.
The main challenge in enriching these clonotypes from within the vast immune repertoire is in analyzing the diversity.
TCR Profiling and TCR Repertoire Analysis
Current immunotherapeutic biologics are successful in less than 30% of patients. The consensus is that responders to immune-biologics, such as immune checkpoint inhibitors, bear a broader and more expanded repertoire of tumor specific T cell clonotypes. As a result, a hot topic and challenge is to study T cell repertoires in responders and non-responders.
Why is TCR Profiling Important?
The specificity of each T cell is mediated through the TCR, which is unique to each T cell. There is a large diversity of TCRs that recognize and eliminate specific antigens. Measuring the diversity of T cells from a tissue sample, provides useful information to help track the responsiveness of a patient’s immune system to specific antigens for effective treatment.
For immunotherapy and/or T cell therapy, it’s critical to track the responsiveness of T cell clonotypes to the specific antigens over time, in different tissues within the patient.
Several different approaches to analyze TCR repertoires exist, but a standard or best practice has not yet been identified. The method used depends on personal preference, end goal, and technical feasibility.
A sequencing approach is one of the earliest adopted methods, and more widely used, as it can be easily outsourced to a contract research organization with DNA/RNA samples. However, there are advantages to some newer approaches.
TCR Analysis Using Next Generation Sequencing-based 5’RACE (Rapid Amplification of cDNA Ends)
Commercial companies offer these TCR analysis services (such as SMART®), using starting materials such as genomic DNA or RNA. This can be performed in bulk repertoire or for a single cell, allowing for analysis of millions of TCR in a single experiment.
While this approach can readily generate results in a high-throughput manner, it requires a large amount of input nucleotides (50-100ng per primer set) and can be costly. Another major challenge organizing groups of TCR sequences according to their predicted antigen specificity.
The Advantages of Using MHC-Tetramers in TCR Analysis
TCRs detect antigens only when peptides are bound in the groove of peptide-major histocompatibility complex (pMHC) molecules on the surface cells. The TCR–pMHC interactions dictate the selection, development, differentiation, fate, and function of a T cell.
Due to the low binding affinity of pMHC monomer to TCRs, pMHC tetramers have been developed, which exhibit 100-fold higher affinity to TCR binding. This provides an ideal candidate to study TCR repertoires. The pMHC-tetramer is a complex that consists of four fluorophore-labeled MHC monomers, each bound to a specific peptide.
Fluorochrome-Labeled MHC Peptide Tetramers
Many fluorochrome-labelled pMHC-tetramers are available. These allow for single cell phenotyping, and enable measurements of different T cell populations simultaneously, with up to 17 colors depending on the flow cytometer.
This method allows for recovery and downstream studies via cells sorting, in comparison with sequencing, where it is impossible to conduct downstream analysis once the cells are being analyzed.
The limitation to fluorochrome-labelled pMHC-tetramers is the requirement to have some knowledge of the specific peptide-MHC involved, in order to select the appropriate tetramer.
DNA-Barcoded MHC Peptide Tetramer
A recent innovation is to tag (or barcode) MHC tetramers with unique DNA sequences The DNA-barcoded complex is identified by PCR followed by sequencing for the specific barcode.
This approach directly identifies, quantifies, separates, and sequences the TCR of tumor specific CD8+ T cells. DNA-barcoding also potentially negates any limitation in using hundreds of pMHC tetramers for T cell repertoire and TCR analysis.
In addition, the advances made in techniques for deep sequencing of TCR molecules has enabled investigators to obtain TCR sequencing information in large numbers of T cells. As a result, researchers can either isolate peptide specific T cells in bulk or at the single cell level using MHC tetramers and obtain TCR sequencing of T cells.
Such platforms are used in bulk or in a single-cell setting. They allow for simpler identification of antigen-specific T cells that are involved in CTL-mediated tumor killing following tumor antigen stimulation.
Identification of Single T Cell Populations by Barcoded pMHC Tetramers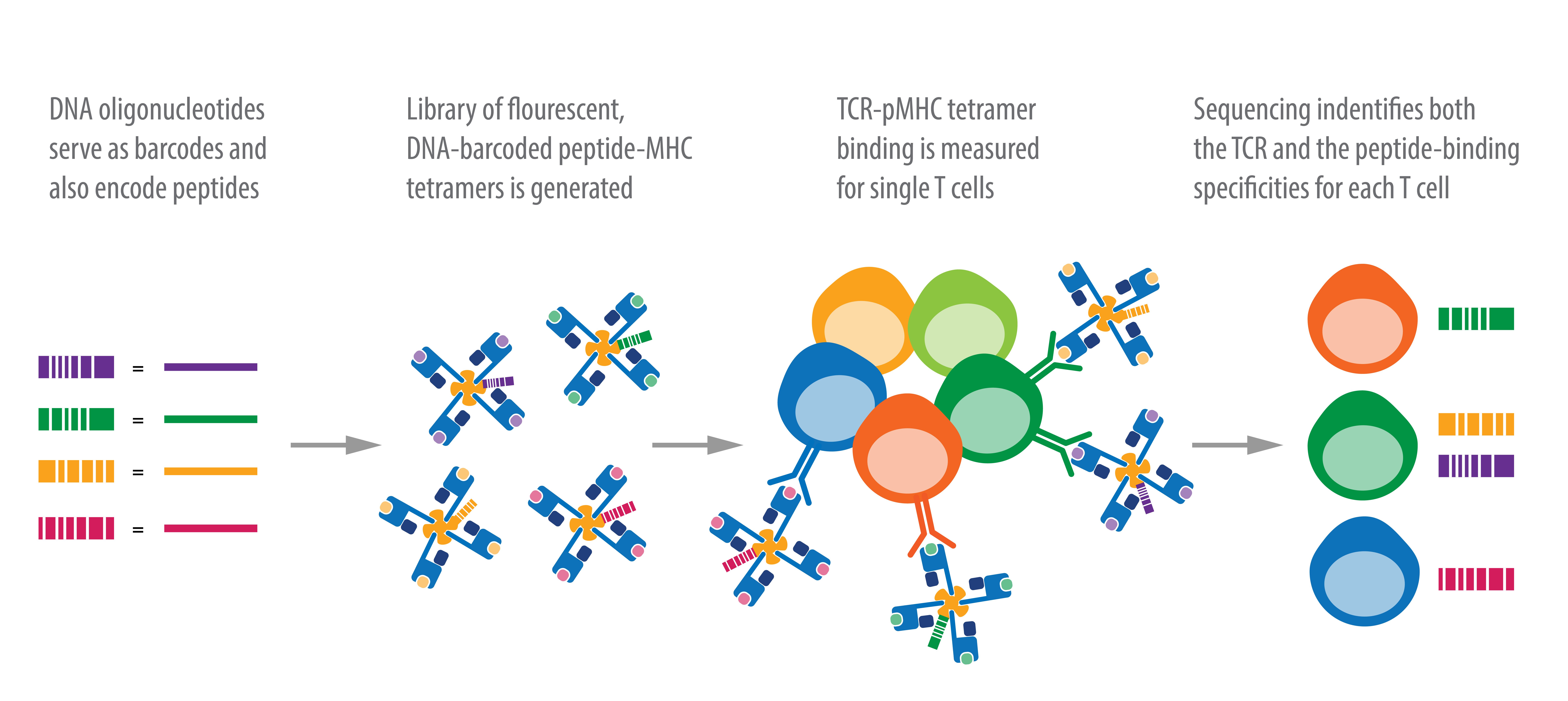
Summary
TCR analysis is key to identifying the specific T cell clonotype that is responsible for tumor clearance following immunotherapy. The recent advances in MHC tetramers has made TCR profiling much easier. This is an otherwise challenging task, given that each T cell contains its own unique TCR sequence that recognizes its corresponding MHC. By profiling the TCR, we can identify the specific antigens that these T cells are targeting.
MHC-tetramers allow investigators to study and dissect tumor specific T cell differences in immunotherapy responders vs non-responders. This technique also identifies the T cell clonotypes and corresponding epitopes (or neoepitopes) that have more impact on tumor eradication. With this information, we can significantly improve downstream design on cancer vaccines or T cell therapies.