Here’s what you need to know about PDX and what to expect from them in your research.
Most preclinical researchers are aware of patient-derived xenograft models (or PDX), but many are still unsure exactly what to expect from these models.
- What added value do PDX offer over traditional xenografts?
- How are PDX different from CDX?
- When are they most useful in your drug development program?
 What are PDX and How are they Different from Cell Line Derived Xenografts?
What are PDX and How are they Different from Cell Line Derived Xenografts?
In drug discovery, animal models are used to understand the efficacy, PD, PK, metabolism, and tolerability of candidate drugs. In oncology, these have traditionally been cell line xenograft (CDX) models using tumor cells immortalized in vitro from patient tissues. These cell lines have been adapted to grow on plastic, used for many generations, and are often greatly altered from original disease.
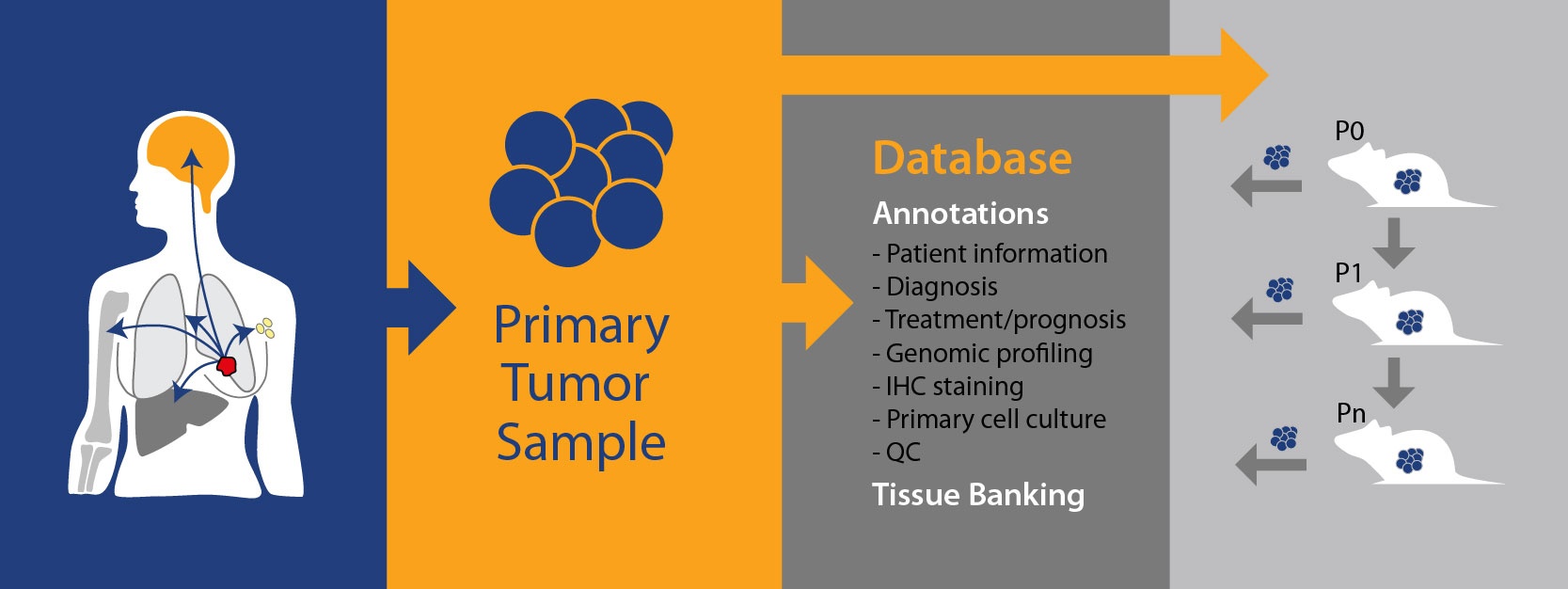
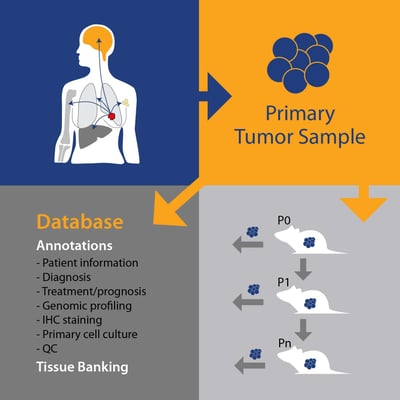
PDX, on the other hand, are generated by taking patient tumor tissue and directly implanting into a mouse, never growing in vitro. Subject to the implanted tissue “taking” in the host and achieving reasonable growth rates, the tumor tissue is subsequently passaged into further animals (P1, Pn, etc.) and becomes a PDX model. These models are constantly maintained in vivo, with heterogeneous cell types and a tumor microenvironment closely mimicking that of human tumors.
What Advantages do PDX Offer?
PDX provide stable models which more closely recapitulate the characteristics of the parental tumor than traditional xenografts – such as histo- and molecular pathology, driver mutations, and oncogenic changes – offering a more predictive alternative for preclinical drug evaluation.
How Do I Know a PDX Model Truly Reflects the Patient Tumor?
Original PDX models can be QC’d (as well as other, low-level passage numbers) to ensure the original tumor pathology is maintained. Similarly, genetic fingerprinting (STR) of models across passages can be performed.
Using only low passage number models (while banking master samples for further model creation) should ensure the lowest likelihood of genetic drift over time.
What Information Can I Expect with a PDX Model?
PDX come with a wealth of both patient and model information. For the patient, this can include background data such as:
- Ethnicity
- Age
- Smoking Habits
- Stage and Grade of Disease
- Biopsy Site
- Pathology Diagnosis
- Prior Treatments, if any
PDX model annotation starts with a pathology QC and model fingerprinting. This is then expanded to cover all the genetic data needed to select models and understand response vs disease background – such as gene expression, copy number, mutation, fusion, and microRNA expression. Data is likely to come from a combination of next generation sequencing (e.g. RNA sequencing, whole exome sequencing) as well as microarray data and model hotspot analysis, verified by in vitro assays as needed.
Immunohistochemistry data is also common for certain disease types, e.g. breast cancer, where a model’s ER, PR, and HER2 expression status is important to determine cancer subtype.
The robust growth needed for efficacy studies is usually confirmed by growth curves, which can also show variability in growth for a certain model. These can be combined with any available treatment data to see how the model responds to SoC or experimental agents, if models are treatment resistant, and to explore response variability vs. background.
Certain disease types should also be available with specific information. With blood cancer models such as AML, for example, you could also expect to find patient primary blood test results, primary marrow morphology, and model phenotyping for antigen expression.
How do I Choose a PDX Model?
Do you want a triple negative breast cancer model, a model harboring a specific fusion, or a SoC-resistant model?
The easiest way to find exactly what you are looking for is through an online collated database of models which allows searching of any model parameter. This could be within one indication, or for high gene expression across a range of cancer types.
Tumor microarrays can also be useful if you want to rapidly check a lot of models for expression of a protein target.
When would I add PDX to my Drug Development Program?
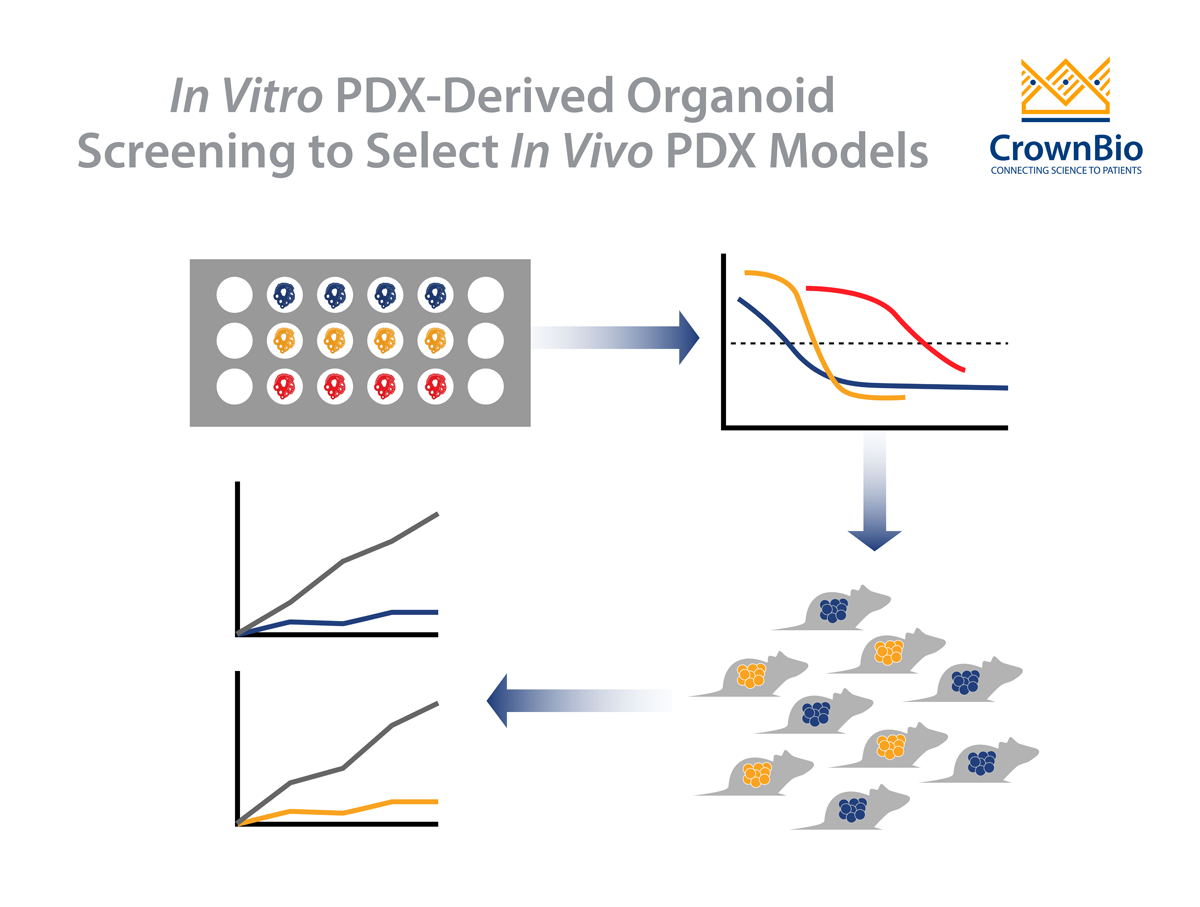
PDX are the ideal tools to use when drug development programs are gearing up for clinical trials, as they have been shown to be the most predictive xenograft model available. PDX growth and response to SoC correlate well with patient clinical response, providing highly predictive data for guidance on indication or patient clinical stratification.
Traditional xenografts are still extremely useful for early stage drug development, especially for the initial evaluation of multiple potential candidates or a compound series, or to evaluate and rank efficacy against a known target which a particular cell line may be known to express.
What are PDX Main Uses?
PDX have a wide range of uses based upon their similarity to patient tumors and predictability of response:
- As individual models to study specific tumor response (based on models harboring specific genetic features you are targeting)
- In population studies for preclinical drug screening, biomarker analysis (including for clinical trial stratification), and identification of sensitivity/resistance mechanisms.
Can I use PDX for Immuno-Oncology Studies?
Yes, provided you implant in humanized mice. PDX are actually an ideal model for immunotherapy studies as they can capture the characteristics and diversity of the human clinical oncology population. Tumors may also use different mechanisms to interact or interfere with the immune system, which PDX could also recapitulate.
If you have any further questions on PDX models or how to use them, or are interested in learning more, please contact us.