 As covered in our recent blog post, Mouse Clinical Trials using patient-derived xenograft (PDX) models provide more predictive outcomes during preclinical studies, hopefully resulting in an improved success rate in clinical trials.
As covered in our recent blog post, Mouse Clinical Trials using patient-derived xenograft (PDX) models provide more predictive outcomes during preclinical studies, hopefully resulting in an improved success rate in clinical trials.
Once you’ve decided to run a Mouse Clinical Trial, the next decision is which type of MCT would be best for your drug development program. This post takes a look at the different types available and what they are useful for.
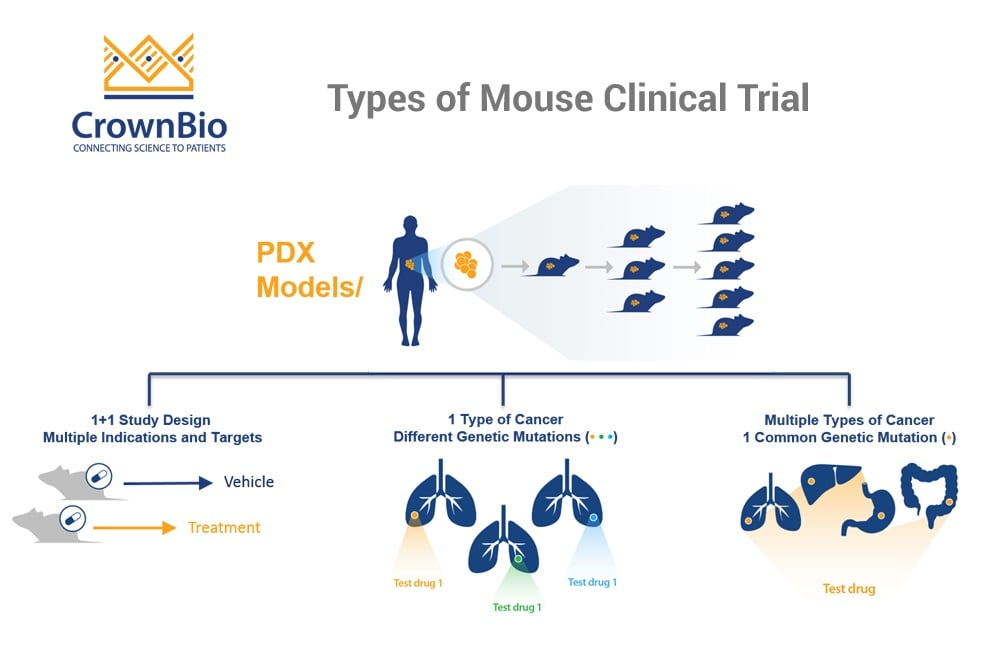
1+1 and 0+1 MCTs Better Mimic Human Clinical Trials
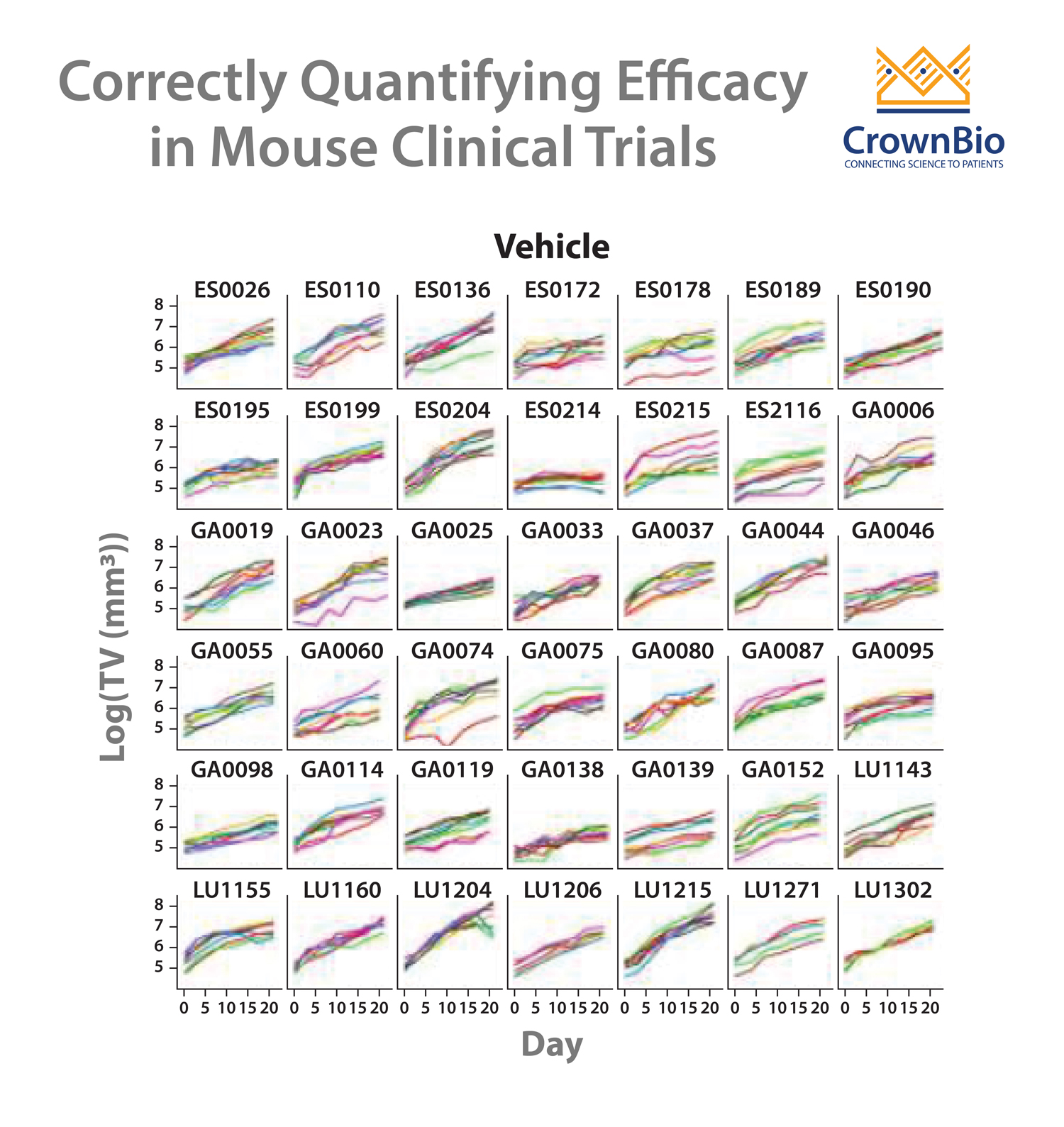
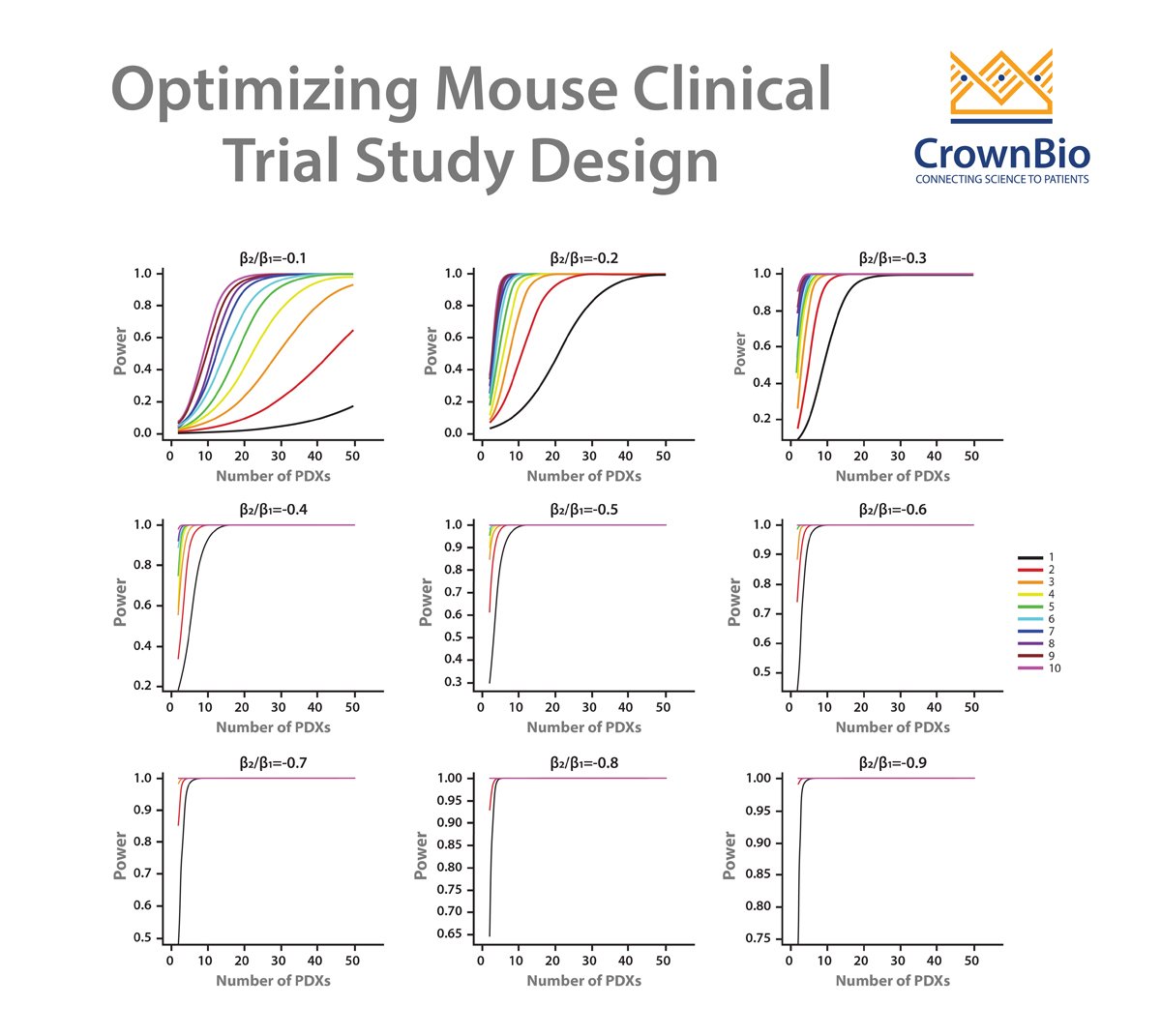
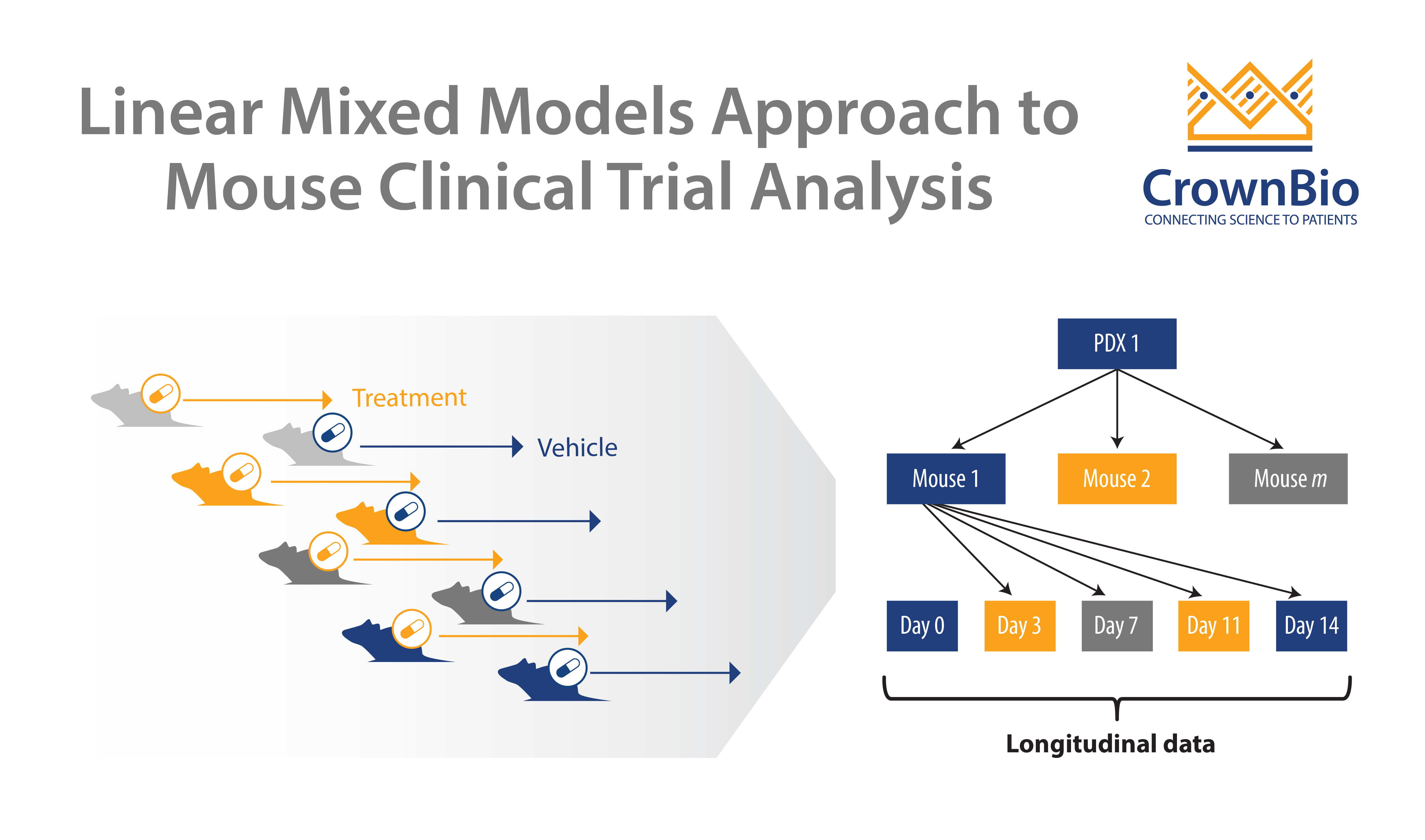
In general, Mouse Clinical Trials use a large number of models with a small number of subjects in each arm. This more closely mimics human clinical trials than traditional preclinical studies, which would use a small number of xenograft models, with a large number of subjects in each arm.
The large group of PDX are each derived from an individual patient, therefore representing the clinical cancer population heterogeneity. Each model receives the treatment or regimen being tested, and the required outcomes are measured.
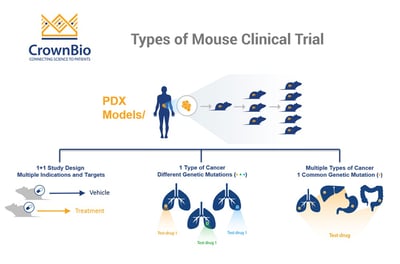
The main first question to ask is whether you want to include a comparator arm (running a 1+1 study) or not (so running a 0+1 study) to more closely mimic Phase II or Phase I trials, respectively.
1+1 MCTs Provide a More Robust Study, Mimicking Phase II Trials
MCTs are often called Phase II-like studies, and the 1+1 design fits that need exactly. Each model has a control/comparator arm, which can be compared with the treatment arm, to provide an easy determination of “response”. The comparator arm is likely to be the current standard of care (if the treatment under evaluation is something like SoC + Agent X.
Having a comparator arm provides a more robust study than if the control arm is removed, though 0+1 MCTs designs are still useful.
0+1 MCTs Mimic Phase I Trials
If you are looking to mimic more of an initial Phase I trial then removing the comparator arm and running a 0+1 study may be the correct approach. Without a control model for comparison, response is evaluated by some form of RECIST criteria e.g. looking at tumor growth or reduction. The removal of comparator arm means a reduced amount of animals, which can also help in minimizing costs.
These trial set ups are then taken forward into indication and target driven MCTs, to test different hypotheses before clinical trials.
Indication Driven MCTs – Mimic Umbrella Trials Looking at One Cancer Type
As precision and personalized medicine continues to expand, clinical trials have undergone many changes, including introducing new methods such as umbrella and basket trials.
An umbrella trial is designed to look at the effect of different drugs on different mutations within one cancer type (“under the umbrella of one disease”). Patients within one cancer type are selected based on their most prominent genetic mutation and can then be treated with a range of new agents to target this, creating subgroups which best respond to each drug.
The indication driven MCT follows the same principle – looking at whether an agent works in only one specific type of cancer, which may be driven by a range of different mutations.
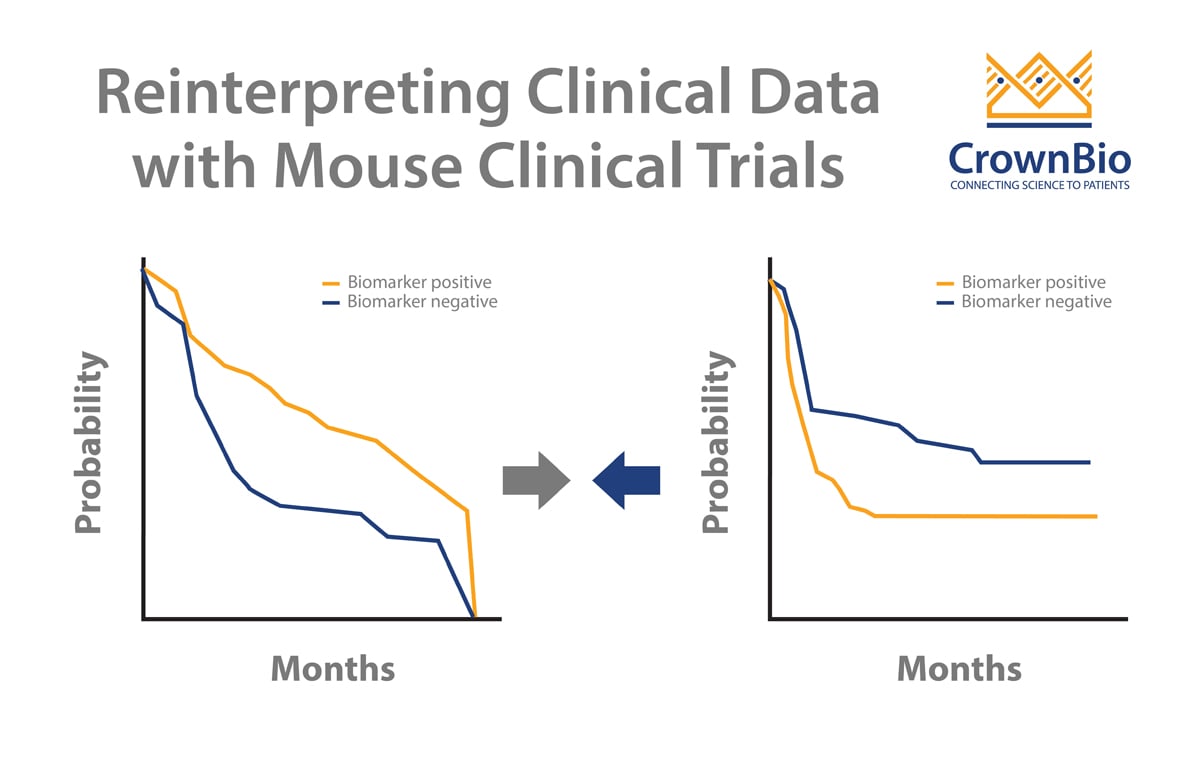
One benefit of this type of Mouse Clinical Trial is that it provides a perfect framework for biomarker discovery within your cancer type of choice, and predictive biomarkers are becoming ever more important for drug approval in personalized medicine.
Inherent in this type of MCT design is that you can’t study agents across many indications, which may be needed to find exactly which indication is the best to move into clinical trials.
Target Driven MCTs Provide Robust Target Validation
If you have an agent and one specific mutation to target and would like to find optimum indications to treat, then a target driven MCT is a better approach. This is similar to a basket clinical trial, where multiple arms (or ‘baskets’) group patients from a range of cancer indications but with the same mutation to test novel targeted therapies.
The target driven MCT is similarly independent of cancer type, including any cancer with the mutation of choice. This method allows thorough and robust target validation – confirming if a mutation is present across a range of cancer types, looking at whether the drug engages the target, and then what happens downstream.
This MCT type won’t support robust target indication selection, as you are likely to explore many different cancer types.
Choose MCT Type Based on Hypothesis to Test
With the wide range of new agents being evaluated today, one preclinical platform is not likely to be useful for them all. Given the flexibility of MCTs, and the different types which can be chosen dependent on the hypothesis you need to test, the Mouse Clinical Trial can therefore expand its usefulness across a wide range of preclinical testing.