Drug resistance to cancer therapy is a significant problem facing patients. It has been identified as the major factor limiting greater treatment success.
The problem is multifaceted, but one path for overcoming drug resistance and targeting drug-resistant cancers is to leverage cancer drug-resistant in vivo cell-derived xenograft (CDX) models.
In this post, we explore how such models can help researchers better understand the mechanisms of drug resistance and support the development of combination or next-generation cancer therapies.
The Problem of Cancer Drug Resistance
Cancer is a highly dynamic disease with multiple determinants that can lead to drug resistance. While therapeutic strategies have certainly reduced the consequences of drug resistance, additional approaches to challenge resistance are still urgently needed.
For example, non-small-cell lung carcinoma (NSCLC) patients with EGFR-activating mutations (i.e., exon 19 kinase domain deletions) often acquire resistance to EGFR tyrosine kinase inhibitors (TKIs). This generally results in disease progression after one year of treatment with first- or second-generation EGFR TKIs.
Although this has led to the development of multiple generations of EGFR TKIs, drug resistance continues to affect even third-generation TKIs. Relapsed patients often develop a secondary EGFR mutation (T790M) that inhibits TKIs from binding to EGFR. This is in contrast to the fewer than 5% of relapses involving mutations in PIK3CA (a downstream signaling lipid kinase).
Despite the challenges, researchers continue to make great progress by studying the mechanisms behind acquired resistance, to develop and test therapeutic strategies that exploit weaknesses of drug-resistant cancers. We will explain below why in vivo cancer drug-resistant models provide a strong platform for preclinical efficacy testing.
Options for Drug-Resistant In Vivo Models for Preclinical Efficacy Testing
Before selecting a drug-resistant in vivo model, researchers should consider the type of xenograft best suited for their study objectives.
As described in a previous blog, patient-derived xenograft (PDX) models are very useful for studying cancer drug resistance because they closely recapitulate the phenotypic and genetic features of patient tumors and have clinically relevant tumor microenvironments. Researchers can use PDXs with known drug resistance, and alternatively, resistance can be induced via genetic engineering or through repeated in vitro challenges to treatment.
Similarly, CDXs are highly suitable for developing important in vivo drug-resistant models. For instance, first-generation EGFR-TKI-resistant models were developed by establishing a drug-resistant cell line by in vitro selection. These cells were then used to establish a drug-resistant CDX in vivo model.
Specifically, the HCC827 (NSCLC EGFR tyrosine kinase deletion) cell line was repeatedly challenged with increasing concentrations of erlotinib or gefitinib, resulting in two resistant cell lines (HCC827-ER1 and HCC827-GR1).
Characterization of these new cell lines showed that both had increased copy numbers of c-MET and expression of Axl compared to the parental HCC827 cells. In vivo validation of drug resistance was confirmed by subcutaneously implanting the HCC827-ER1 cell line into nude mice (MF1-nu/nu).
In Figure 1, erlotinib was very effective in inhibiting tumor growth in “wild-type” cells (yellow plot) but much less effective in preventing the growth of the HCC827-ER1 CDX (blue plot).
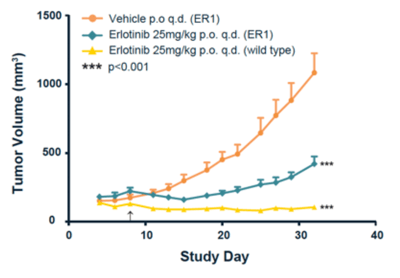
Figure 1: In vivo validation of the HCC827-ER1 drug-resistant cell line. Source: Crown Bioscience “HCC827 NSCLC Cell Line Derived Xenograft Model”
An in vivo combination therapy confirmed c-MET amplification as the mechanism of resistance for HCC827-ER1. This cell line has been used in several published studies (here and here, for example) on mechanisms of resistance and/or new drug candidates.
FDA Approval Supported by Drug-Resistant In Vivo Models
Rybrevant is the first FDA-approved targeted treatment for patients with NSCLC with EGFR exon 20 insertion mutations. This medication provides patients with a new option to combat drug resistance, and the evidence package supporting the approval of this drug included key in vivo drug-resistant models described below.
Researchers from Janssen Research and Development tested the efficacy of the novel bispecific antibody (JNJ-61186372 [Rybrevant]) against EGFR-TKI-resistant lung tumors.
They found that the antibody effectively bound to both EGFR and c-MET (as expected), and treatment in NSCLC tumor models (including HCC827-ER1) resulted in tumor regression. However, they also reported that some NSCLC tumor models continued to grow, indicating resistance.
In a follow-up study, researchers sought to use proteomics to profile in vivo signaling changes that occur in osimertinib- and JNJ-61186372-resistant tumors from their NSCLC tumor models, including HCC827-ER1. This new approach enabled them to identify tyrosine phosphorylation rewiring as a mechanism of resistance. They noted that this finding would not have been possible using cell population averages from in vitro analyses.
Conclusion
Cancer drug resistance remains a major challenge in oncology. By leveraging drug-resistant models, such as the HCC827-ER cell line, preclinical studies can investigate new mechanisms of resistance and test new strategies and treatments, including combination or next-generation therapies.
To read Crown Bioscience’s full application note on drug-resistant HCC827, please click here. You can learn more about our CDX models here.