 The future of cancer treatment is often considered to be combination regimens (dual immunotherapeutics or immuno-oncology + targeted agents), which is driving the need to develop models for the assessment of human-specific agents and combinations prior to clinical trial.
The future of cancer treatment is often considered to be combination regimens (dual immunotherapeutics or immuno-oncology + targeted agents), which is driving the need to develop models for the assessment of human-specific agents and combinations prior to clinical trial.
Last year we took a brief look at stable and transient humanized mouse models, as well as humanized drug target GEMM models, which can all fulfil the role of testing human origin agents. This post comes back to humanized mouse models, now taking a closer look at hPBMC vs hCD34+ mice, from how their development differs, how this affects model features and limitations, and how combining these with problem driven model selection can help you choose the correct model to use for any human-specific agent immuno-oncology study.
Different Engrafted Cell Populations Results in Two Different Humanized Mice
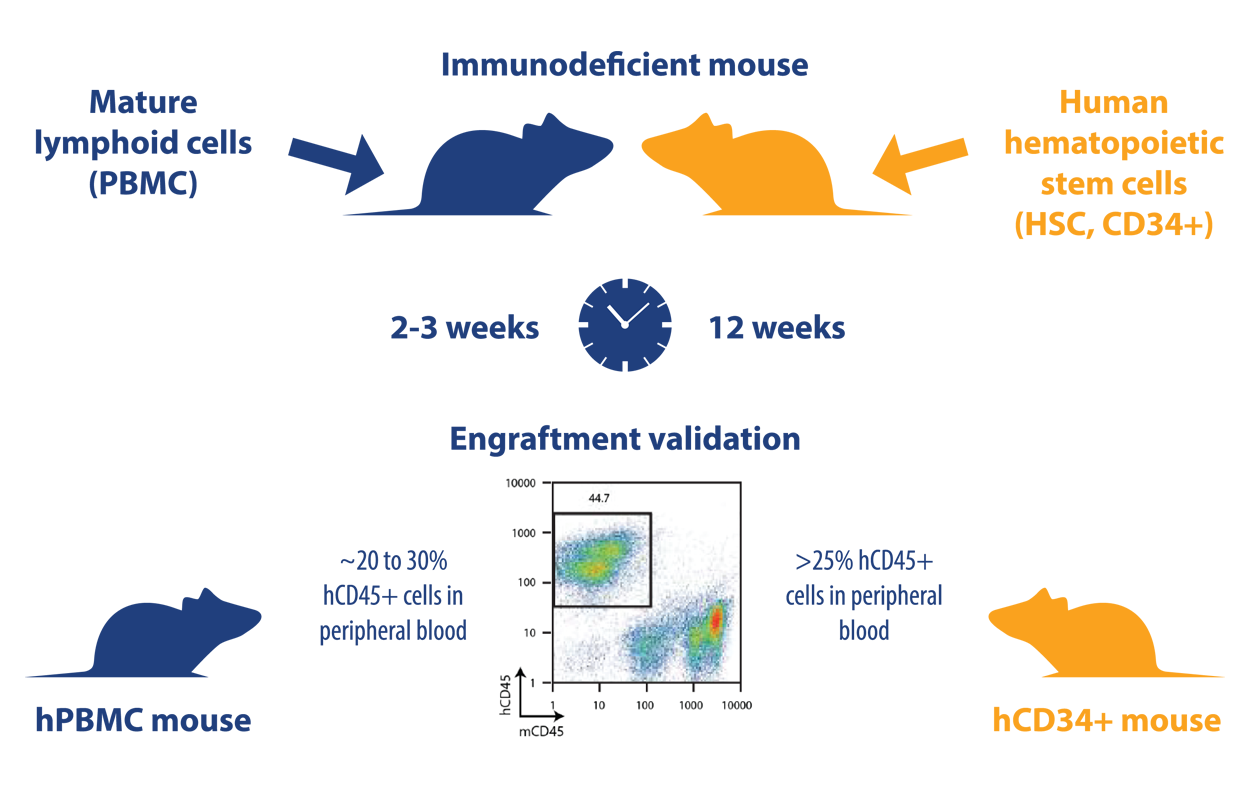
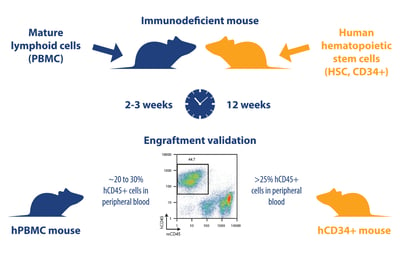
hPBMC (transiently humanized) and hCD34+ (stable humanized) models are generated in a very similar way. The main difference is the cell populations used to create the models:
- hPBL (peripheral blood leukocyte) models: generated by injection of mice with mature lymphoid populations including peripheral blood mononuclear cells (PBMC), lymph node cells, and splenocytes. For immuno-oncology studies, PBMC are the most commonly used source of PBL
- hCD34+ models: generated using human CD34+ hemopoietic stem cells (HSC), also called Scid repopulating cells, derived from bone marrow, umbilical cord blood, fetal liver, or G-CSF-mobilized peripheral blood
Each population is injected i.v. or i.p. into immunodeficient mice (for hPBMC models, leukocytes can also be administered subcutaneously admixed with tumor cells), and then validated by FACS at an appropriate time for levels of hCD45+ cells (usually around 20-30% in hPBMC models and >25% for a successfully humanized hCD34+ model). Engraftment success rates are similar, though slightly lower for hCD34+ mice (95% vs 85-95%).
Based on using mature lymphocytes or hemopoietic stem cells, each humanized mouse model has different features and limitations which vary model use.
hPBMC Models are Available to Use More Quickly Post-Engraftment, hCD34+ have Longer Timelines
hPBMC models are rapidly available for use – with quick immune cell reconstitution which is validated as early as 2 weeks post-engraftment.
The hCD34+ models are more complex to develop, and are therefore available for use much later based on a variety of reasons. Firstly, the immunodeficient mice need to undergo myeloablation via irradiation before engraftment. This creates a niche in the bone marrow for the stem cells to take and establish themselves. The mice then need recovery time before engraftment can occur. Secondly, immune cell reconstitution takes much longer due to HSC needing to mature and develop into different lymphocytes and myeloid cells (validation by FACS occurs around 12-16 weeks post-engraftment), meaning study initiation can be as long as 15-20 weeks post-engraftment.
hPBMC Models Have a Shorter Treatment Window, hCD34+ Provides More Long Term Model
While hPBMC mice are available to use relatively quickly post-engraftment, they also have short timelines for use. This model type develops graft vs host disease (GvHD) which begins to occur in as little as 2-3 weeks post-engraftment, and really takes hold by week 4.
This means that you potentially have only 15-20 days to fit in your efficacy study, and need to make sure that immune cell reconstitution and tumor growth are optimized – so that reconstitution and appropriate tumor volume occur at the same time to complete any study before severe GvHD manifestation.
hCD34+ models often don’t show GvHD, or if they do it is only minor and asymptomatic with no effect on tumor engraftment, meaning they have no limited treatment window. Instead, they offer a much more long term solution – commonly used immunodeficient mice engraft HSC with high efficiency, and support human immune cell maturation up to 32 weeks post engraftment, providing a wide experimental window.
hCD34+ Models Develop Multi-Lineage Human Immunity, hPBMC More Limited
A highly important difference between these models is the type of immune cell reconstitution which occurs. hPBMC humanized mice are the more simple model - a good reconstitution of T and NK cell lineages can be achieved, whereas B cells are poorly reconstituted.
For the hCD34+ model, stable reconstitution of B, T, and NK cells (as well as myeloid lineages in next generation humanized mice) is observed. However, there is incomplete B cell maturation, which is due to a developmental block in the B cell maturation pathway, stopping B cells from generating sufficient antibody responses. A poor IgG response is particularly seen, with isotype class switching from IgM to IgG not occurring for this model. It should also be noted that human T cells are educated on the murine thymus and are mouse MHC restricted; therefore, T cell reactivity may not be against tumor specific antigens.
Choosing the Correct Model for an Immuno-Oncology Study is Based on the Features Above
Both hPBMC and hCD34+ models are highly useful, and choosing which one to use is usually straightforward, via problem driven model selection based on the immunological features and limitations discussed above.
If you need a model for a short term study, or you are working on short timelines, and your agent has a specific target on human T or NK cells then hPBMC would be ideal to use. This could potentially be initial agent testing or selection before moving to a fully humanized more complex study. The types of agents assessed can include checkpoint inhibitors and BiTE®-like antibodies (which act on T cell functions) or ADCC mAbs and NK modulating agents (for NK cell functions).
Choosing the hCD34+ model is more likely when you need to assess agents which require multi-lineage immunity. As the human immune system is more fully reconstituted, these models are also useful to investigate more specific mechanistic questions surrounding the stimulation or suppression of the immune response. With the wide therapeutic window, hCD34+ mice are also great for long term studies such as assessing memory response and long term antitumor effects.
Donor Variability is an Issue for Both Models – Overcome by Careful Study Design
One topic not discussed yet and important for both models, is donor-to-donor variability, although this can be compensated for with careful study design. In hPBMC models, efficacy varies in mice from different donors, but you can partially overcome this by using PBMC from at least two different donors in each study.
For hCD34+ mice, donor variability is also observed within studies, and if a study needs to be repeated original donors are not available to return to. In this case, variability is usually overcome by evaluating a range of models across a range of donors – a checkerboard design which we have previously looked at in one of our blogs.
Highly Useful Immuno-Oncology Models for Human-Specific Immunotherapy Evaluation
To sum up, hPBMC and hCD34+ humanized mice display many different features and limitations, and looking at these head to head, along with reviewing the questions you need a study to answer, should allow for a straightforward model choice and optimized immuno-oncology study.