Learn about PDX-derived organoids, including PDXO establishment and utility in a pioneering drug discovery platform combining the benefits of in vitro PDXO and highly predictive in vivo PDX models.
PDX-Derived Organoid Models
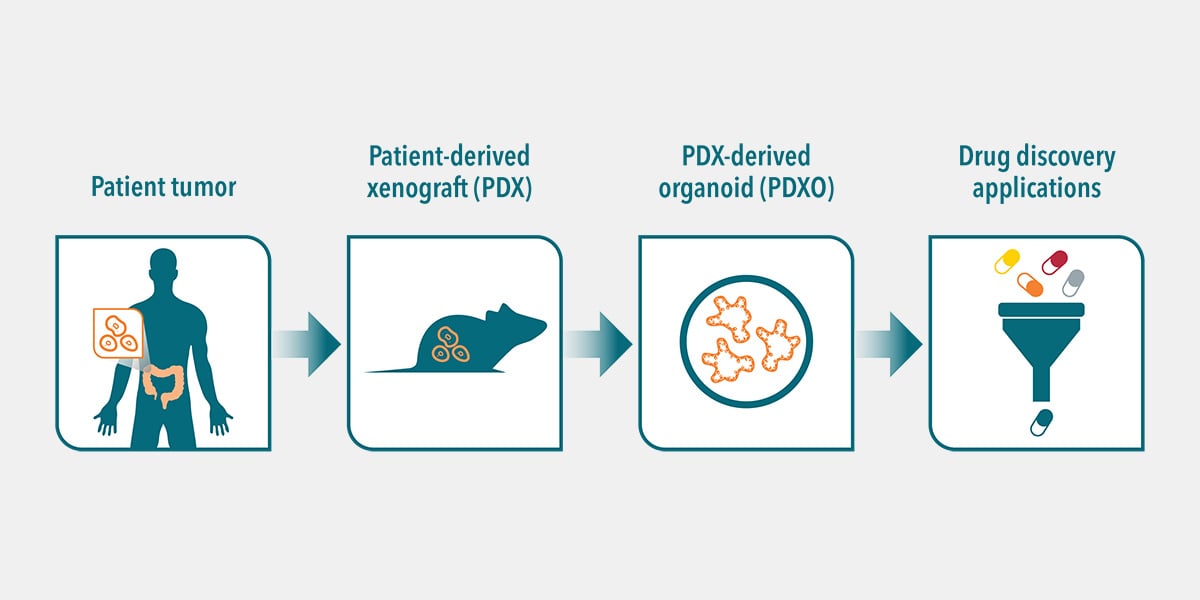
PDX-derived organoids (PDXOs) are 3D in vitro models generated from patient tumor tissue that’s been previously passaged into murine models for expansion. More simply, PDXO are in vitro models derived from in vivo patient-derived xenografts (PDX).
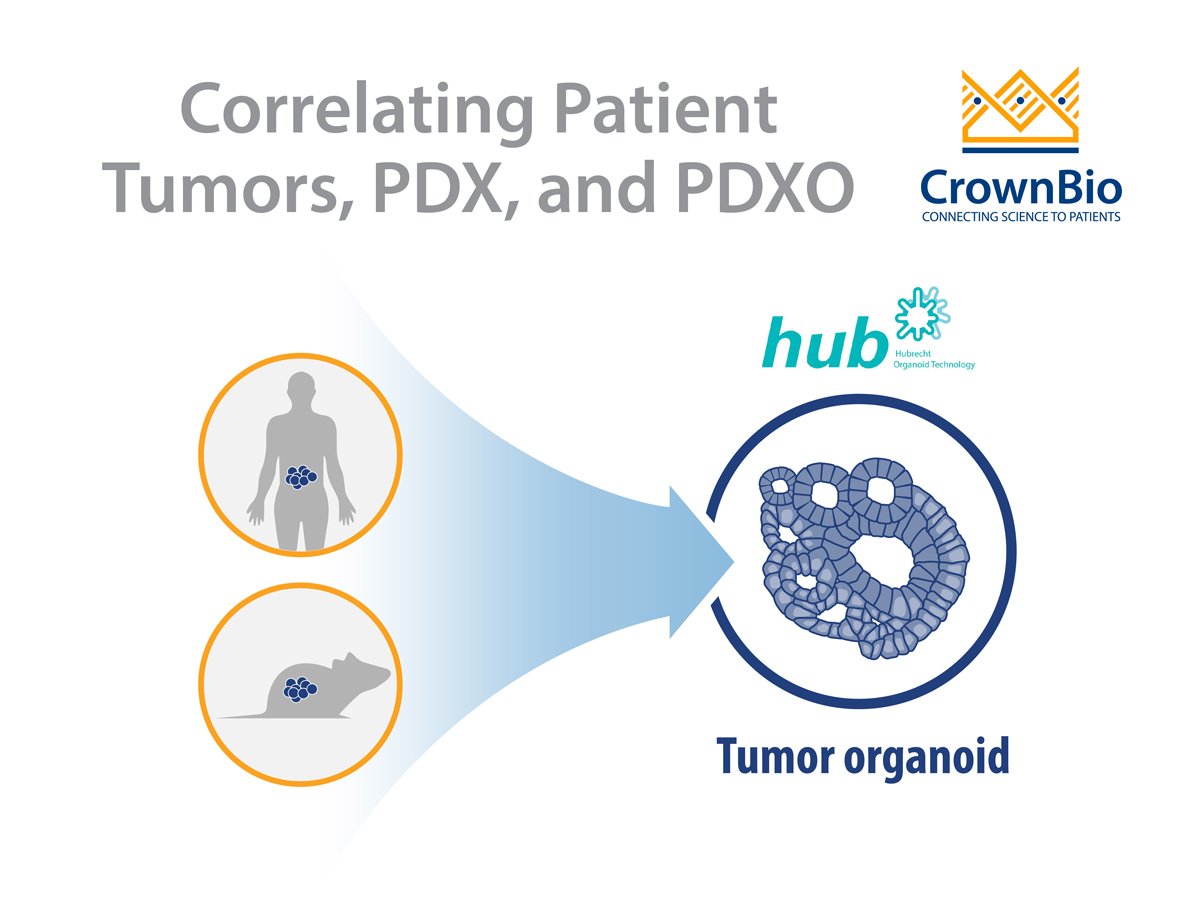
PDXOs are generated using the same protocols as patient-derived organoids (PDO, developed directly from patient tumor tissue). For both models, cancer stem cells (CSCs) are preserved and give rise to cancers found in human patients. PDXO derivation methods include protocols from Hubrecht Organoid Technology which are optimized and adapted for the growth of tumor organoids.
Briefly, PDX tumor cells are cultured in a highly enriched growth medium containing a tailored cocktail of growth factors. This allows the long-term expansion of CSCs and their progeny, with high stability and genomic integrity. This leads to multicellular 3D PDX-derived organoids composed of different cell types reflecting those in the original patient tumor.
Since PDXOs derive from cancer stem cells, they faithfully recapitulate the histopathological properties of the corresponding in vivo PDX tumor (features which are lost in conventional in vitro models). PDXO models also retain key genomic and phenotypic features from the patient’s primary tumor. This results in a robust in vitro 3D model for cancer research and drug discovery.
Why are PDX-Derived Organoid Models Needed?
Traditional oncology drug development strategies are failing. Oncology agents have a higher attrition rate than other therapeutic areas, with drug efficacy rather than safety being the clinical stumbling block.
There’s a clear need for advanced in vitro models with improved predictive power, and to guide more relevant in vivo model selection. Choosing more clinically relevant in vitro and in vivo models as early as possible will accelerate drug development, and make preclinical programs more cost-effective.
Importantly, improved models such as PDX-derived organoids can also help researchers develop a more complete understanding of which patient subgroups will respond to a therapy, and the underlying reasons why.
Developing a platform combining robust in vitro PDXO assays with matched in vivo PDX model response offers a unique preclinical workflow to translate preclinical efficacy findings into clinical success.
The Value of PDXOs in Drug Development
To fully understand the value of PDX-derived organoids in vitro, it’s important to first understand the value of PDX models in vivo.
PDX are the best preclinical in vivo model for predicting clinical outcomes. This is because PDX faithfully recapitulate the genomic and phenotypic complexities observed in cancer tissue. As PDX are derived directly from patient tumor tissue (and never propagated in vitro on plastic), they show high genetic integrity relative to the original patient tumor. Panels of PDX models from a patient population also represent that populations’ tumor heterogeneity.
Large collections of PDX models already exist for many different tumor types, including breast, colorectal, and lung cancer. These models are very well-characterized, with gene expression profiles, in vivo pharmacology, and protein biomarker detection data available. This means that large libraries of PDXOs can be established from available PDXs with relative ease and speed providing the only available in vitro system to display patient relevance.
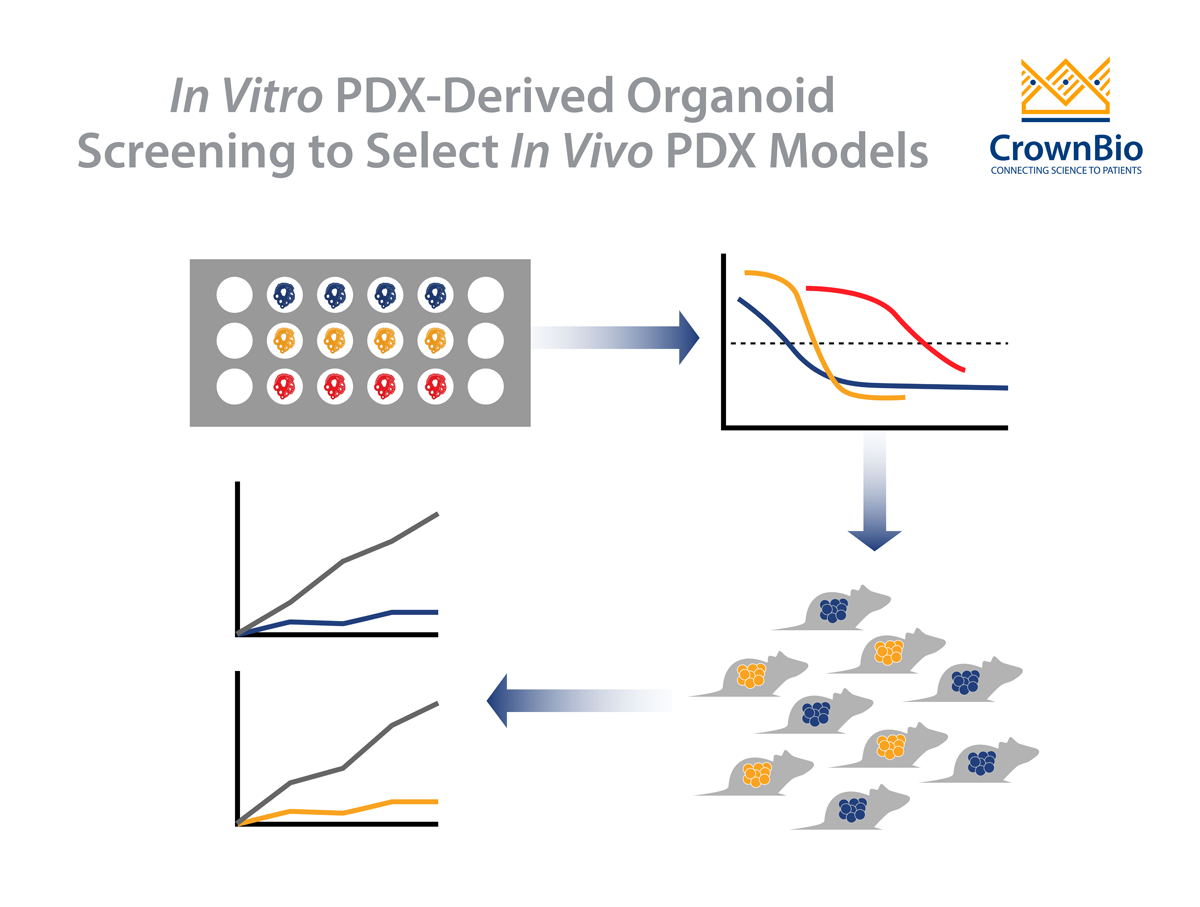
Developing a large library of annotated and extensively characterized PDXO (similarly to their matched PDX), allows us to take full advantage of the panel as a surrogate for the cancer patient population in high throughput drug screens.
Therefore, in developing in vitro PDXOs with matched in vivo PDX counterparts a pioneering drug discovery platform is created, with the potential to dramatically improve preclinical predictivity.
Benefits of PDXOs
PDX-derived organoids offer significant advantages over standard 3D models such as spheroids. PDXO:
- Better recapitulate the original disease (histopathology and molecular pathology).
- Better mimic the original tumor architecture, which is known to influence drug response.
- Provide higher quality read-outs with lower batch to batch variation and high signal:noise ratio.
- Provide improved predictivity of tumor response in vivo.
Compared with in vivo models, PDXO are relatively easier and faster to establish. This means that PDXO are better suited to large scale studies (testing multiple agents and combinations) than PDX. This allows in vivo platforms to instead be used for more focused studies, and results in a cost reduction in preclinical screening.
Applications of PDXOs
As discussed above, PDX-derived organoids provide an easily scalable in vitro system for large scale screens to obtain initial drug sensitivity data. Agents can then be moved to a more complex in vivo system, using matched PDX derived from the same tumor tissue and cells as the PDXO. This allows the refinement and validation of your therapeutic strategy, in the first paired and most predictive platform of its kind.
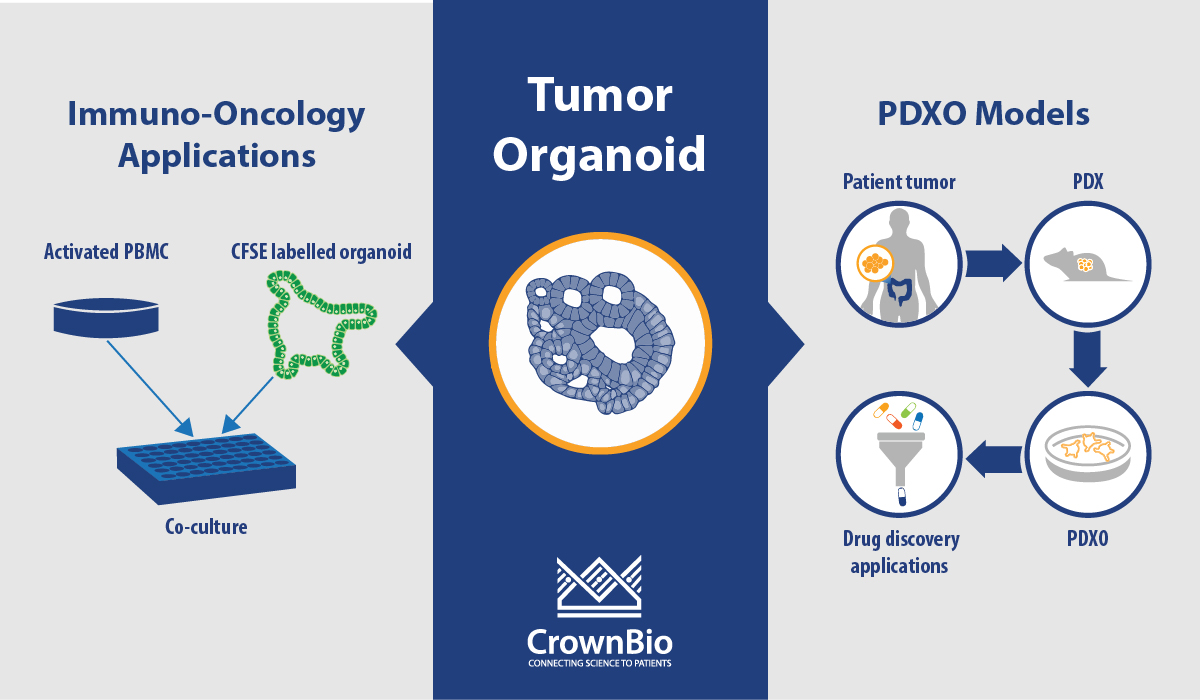
Additionally, all the standard assays and techniques performed on cancer cell lines can also be performed on PDX-derived organoids, providing a versatile in vitro model. With the high level of genomic characterization data available, PDXO also provide a tool for in silico drug discovery.