 Explore how to use a donor selection assay to limit the chances of graft vs tumor (GvT) effects when using huPBMC humanized mouse models.
Explore how to use a donor selection assay to limit the chances of graft vs tumor (GvT) effects when using huPBMC humanized mouse models.
huPBMC Humanized Mouse Models
Human PBMC-transduced models are the most commonly used humanized mice in immuno-oncology studies. In this system, huPBMCs are added to xenograft models either admixed with the tumor cells or the PBMCs are independently introduced intravenously or intraperitoneally.
This results in the murine models being engrafted with human immune cells, primarily T cells. huPBMC-transduced mouse models provide a rapid, simple, and robust alternative to full stem cell reconstitution for testing various human-specific immunotherapies.
huPBMC Humanized Mouse Model Strengths and Limitations
huPBMC-transduced mouse models have a number of strengths which have resulted in their widespread use, including:
- huPBMC-transduced mice are a T cell dependent model, and a significant number of immunotherapies involve T cell modulation/retargeting
- huPBMC humanized murine models are relatively easy to set up and do not need specialized training or capital equipment
- Time to engraftment is very fast
- The same immune cell donor can be used multiple times allowing for longitudinal studies
- huPBMC-transduced mouse models are more cost-effective than other humanized models
Despite this popularity there is still some hesitation around using the model. Two main reasons for this are:
- The high possibility of GvT, which can prevent tumors from growing in this model
- The onset of GvHD in this model which shortens the length of the study
These two issues can be alleviated to some extent by selecting suitable huPBMC donors, as the severity of these two effects are donor dependent. Donor selection to alleviate GvT can be performed with a short-term co-culture assay, while donor selection to alleviate GvHD requires in vivo assays using xenografts of the tumor model(s) of interest.
Donor Selection Assays to De-Risk huPBMC Humanized Mouse Studies
To reduce the risk of GvT in in vivo huPBMC humanized mouse studies, huPBMC donors can be selected that have a reduced allogeneic reaction to the tumor model being used. HLA matching of the huPBMC donor and tumor model has been employed, but there is still controversy as to whether this alleviates the issue.
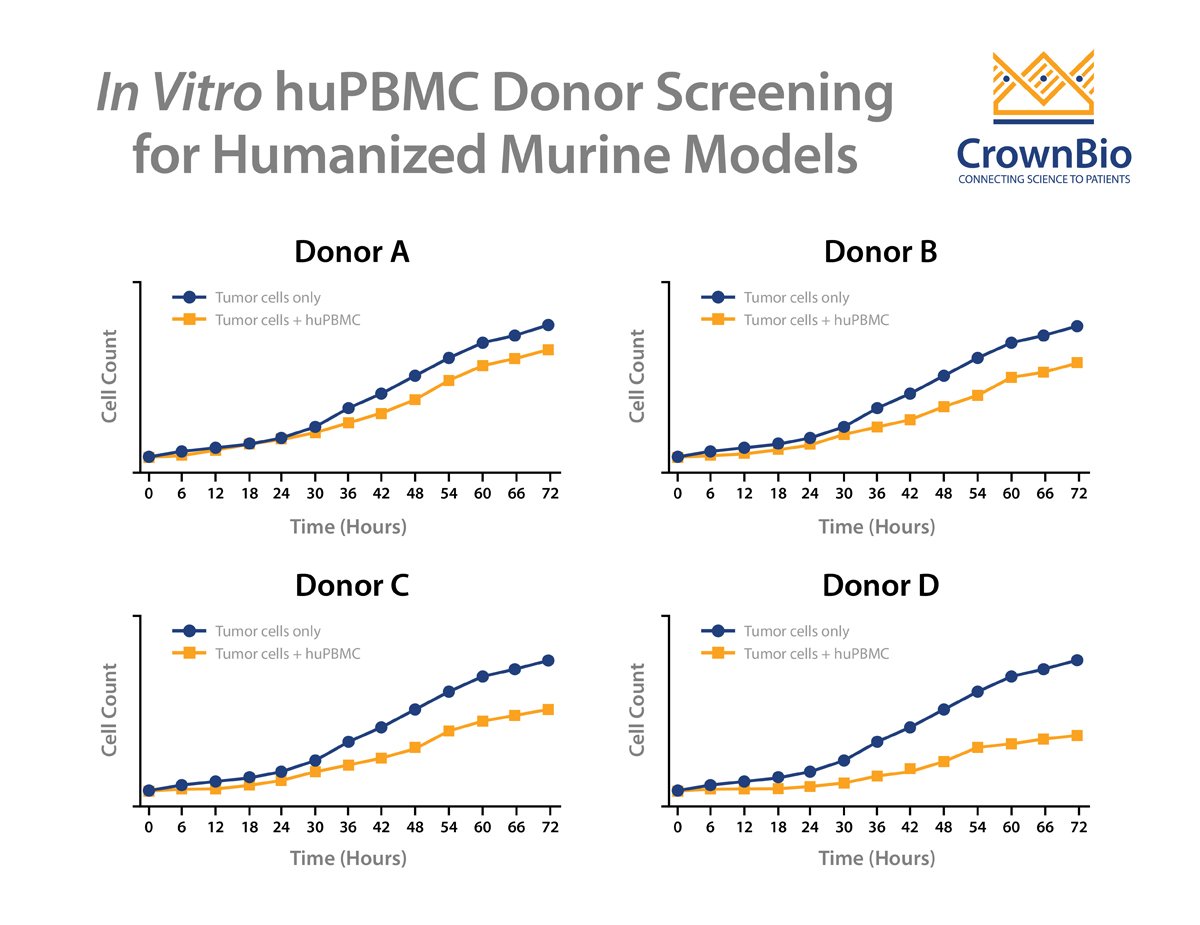
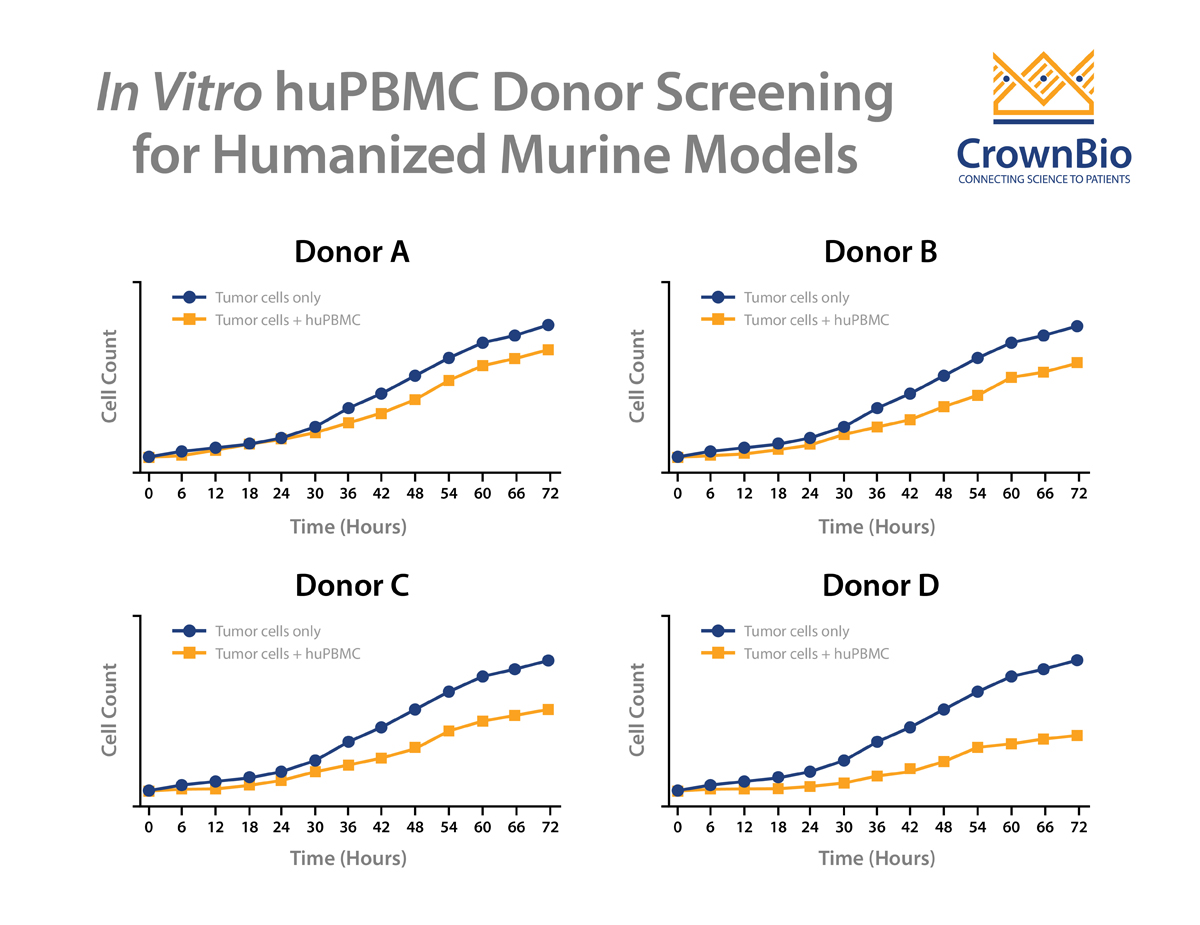
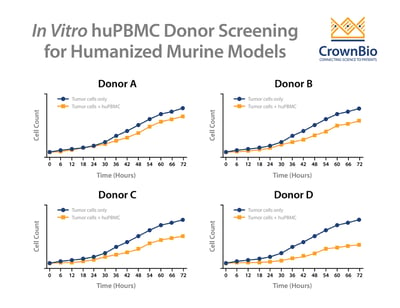
One successful method to reduce the likelihood of GvT is to screen donors by performing an in vitro co-culture between the donor huPBMCs and the tumor cells. To perform this assay, huPBMCs from various donors are seeded with the tumor cells of interest and then co-cultured for up to 72 hours. The allogenic reactivity is assessed by looking at tumor cell killing at various effector-to-target ratios of the huPBMCs.
Multiple donors are then evaluated to see the extent of in vitro tumor cell killing in the absence of specific therapeutic activation. The donor with the least allogeneic reactivity against the tumor is chosen for in vivo studies, as this donor is the most likely of those tested to allow in vivo tumor growth.
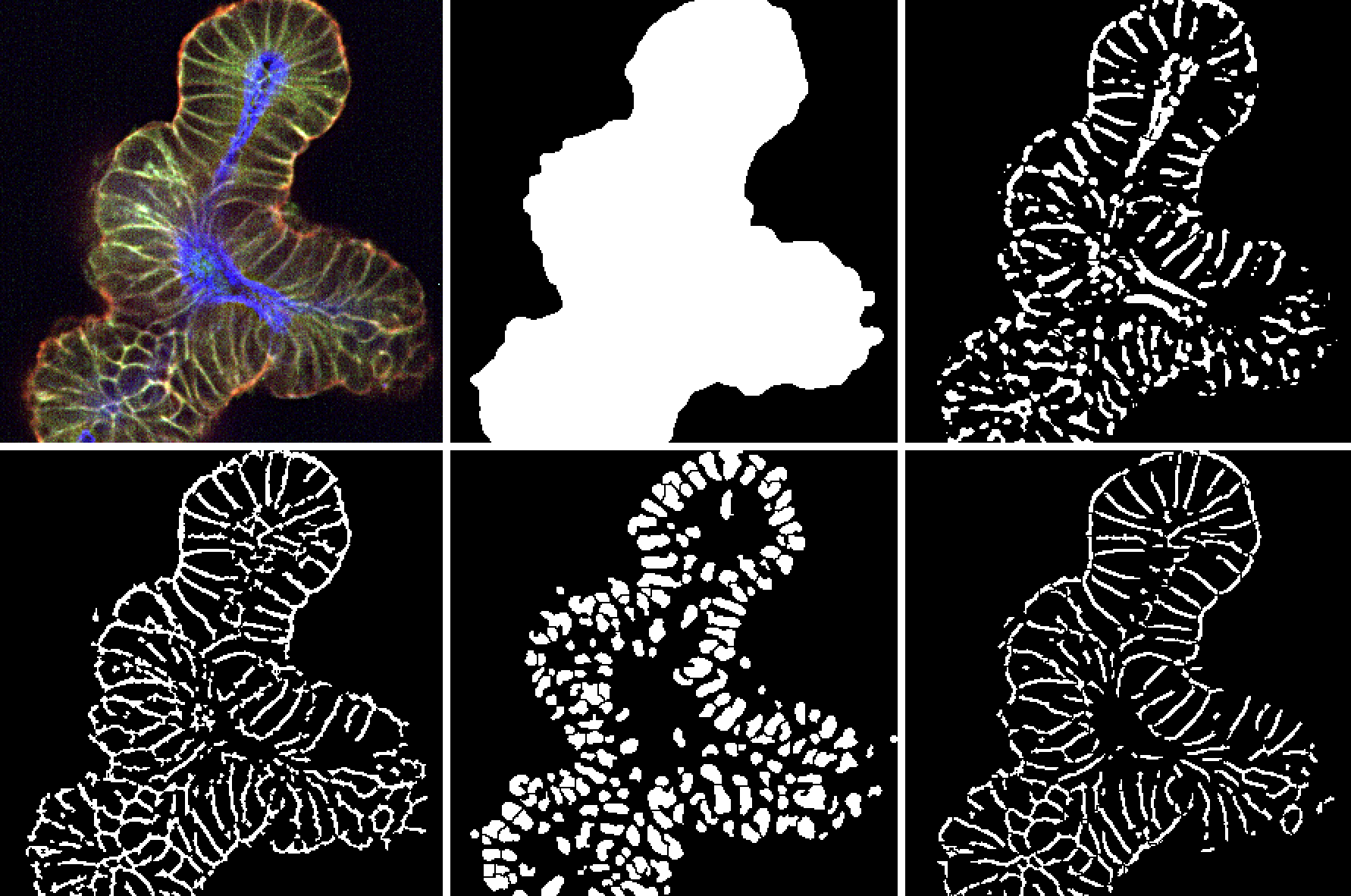
Normally this assay type would require the use of a conventional, cell line derived tumor model, since the typical study involves a 72-hour in vitro cell line co-culture. However, with the development of tumor organoids, patient-derived xenograft (PDX) models can be first converted to organoids and then be assayed this way.
To assess which donors would have the longest delay before the onset of GvHD, pilot studies would have to be performed using xenografts of the tumor model(s) of interest. It is recommended to use donors that have been prescreened in vitro for GvT in the in vivo screen for GvHD. It is also recommended to look at injecting different amounts of PBMCs per murine model. The onset of GvHD can be assessed by monitoring body weight and body conditioning of the models.
Conclusion
Performing pre-screening assays to look at GvT with multiple donors is a successful way to de-risk in vivo studies using huPBMC humanized mouse models. Donor(s) that are successfully pre-screened in these assays can be stored and used for further longitudinal assays, minimizing the number of variables from study to study.