 Explore the current methods of mouse clinical trial analysis, and how these are improved by using a linear mixed models approach.
Explore the current methods of mouse clinical trial analysis, and how these are improved by using a linear mixed models approach.
What are Mouse Clinical Trials?
Mouse clinical trials (MCTs) are increasingly being used in oncology drug development. MCTs are population studies designed to provide predictive preclinical data on subgroups of responders and non-responders to a specific agent or combination regimen. This provides a powerful platform to determine drug efficacy and for biomarker discovery.
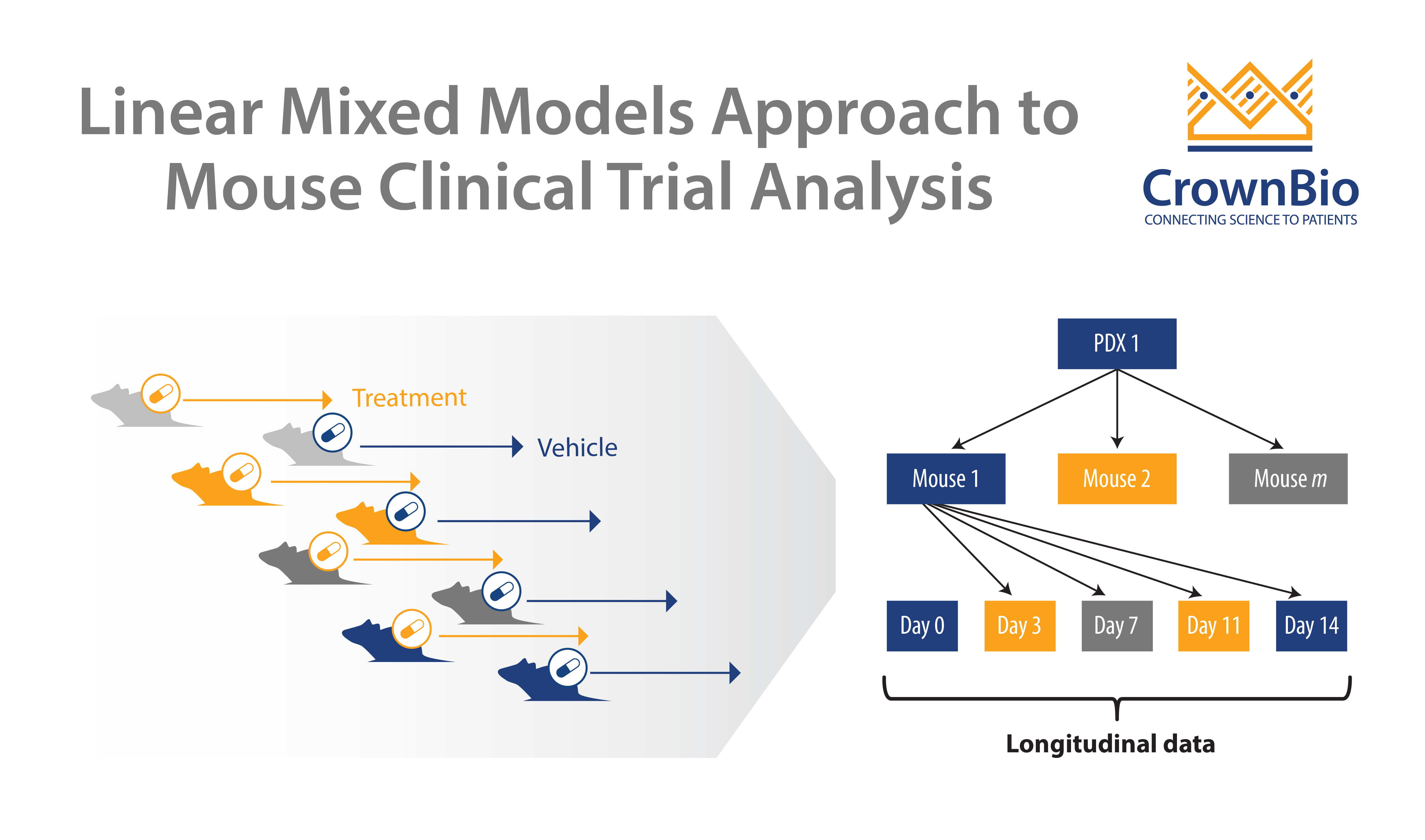
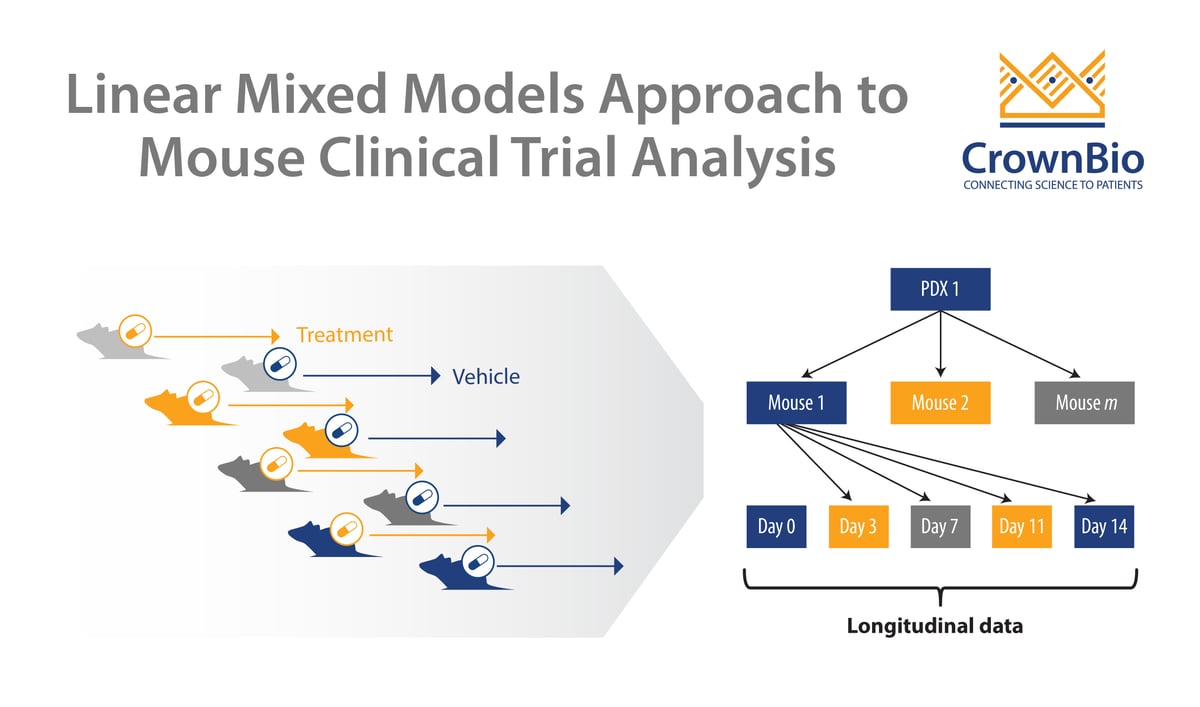
MCTs are modeled closely on human clinical trials, using panels of patient-derived xenografts (PDX) in a randomized, controlled, and statistically powered setting. MCT design mimics the clinical trial setup, using a large number of PDX models with a small number of subjects per arm. This inverts traditional preclinical murine model study design, which usually uses a small number of models with a large number of subjects.
Each PDX model within an MCT acts as a patient ‘avatar’ – meaning the PDX maintains the pathology of the original patient tumor. By using a large panel of PDX within an MCT, the inter- and intra-tumor heterogeneity of the oncology clinical population is replicated.
MCTs have multiple uses across drug discovery in testing a range of hypotheses:
- Hypotheses with a targeted focus, i.e. one target/mutation across many cancer types.
- Hypotheses with an indication focus, i.e. one cancer type with potentially multiple driver mutations.
Study designs can mimic Phase I and Phase II trials to answer many questions on efficacy/response, response and genotype association, biomarker discovery, and exploring mechanisms of resistance.
How Are Mouse Clinical Trials Analyzed?
Similar to human clinical trials, mouse clinical trials are currently analyzed for drug efficacy using endpoint-based approaches. For human trials, metrics include overall survival (OS), progression-free survival (PFS), and objective response rate (ORR; considering both percent complete and partial response).
Some unique endpoints are employed for PDX study analysis:
- Tumor growth inhibition (TGI)
- ΔT/ΔC (tumor volume changes relative to initial volume for drug group and vehicle group)
- Growth rate difference/ratio
- Percent TV change at the end of a trial or on a particular day.
Moving Beyond Endpoint Based Analyses for MCTs
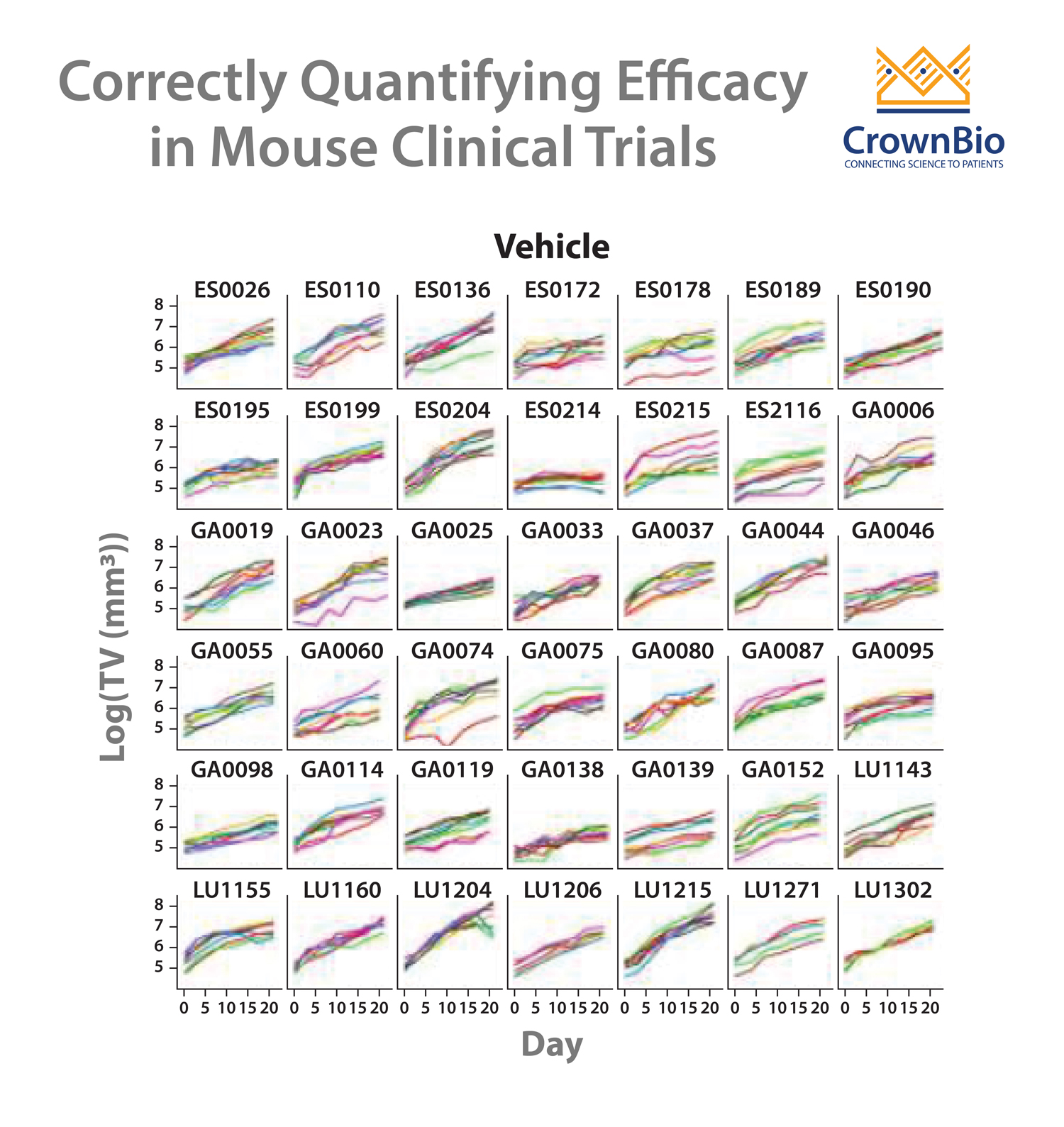
It’s become clear though, that using endpoint-based approaches is not fully appropriate for MCT analysis. While the methods are convenient and valuable in MCTs, they don’t take advantage of the full dataset generated, e.g. tumor growth data or drug response heterogeneity between individual models and mice.
Reducing multiple tumor volume per day data points into a single number such as ORR or TGI sacrifices data. Variability in growth and drug response between PDX (inter-tumor heterogeneity) and between murine models (intra-tumor heterogeneity) is also not considered. MCTs are clustered longitudinal studies, providing additional data that human trials can’t, therefore it’s becoming necessary to develop new approaches to analyze MCTs.
Linear Mixed Models for MCT Analysis
To build a statistical framework that optimizes the full potential of MCT data, first a classical model of tumor growth kinetics based on an exponential model needs to be considered. The data could be log transformed into a linear equation, but this approach may be too simplistic.
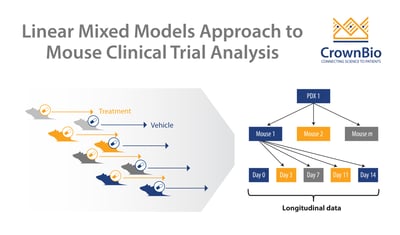
Instead, linear mixed models (LMMs) are now being used to model and explicitly describe heterogeneity in growth and drug response between PDX and between mice of the same PDX for MCTs.
LMM can be applied in multiple ways to address fundamental questions on MCT study design and analysis, through:
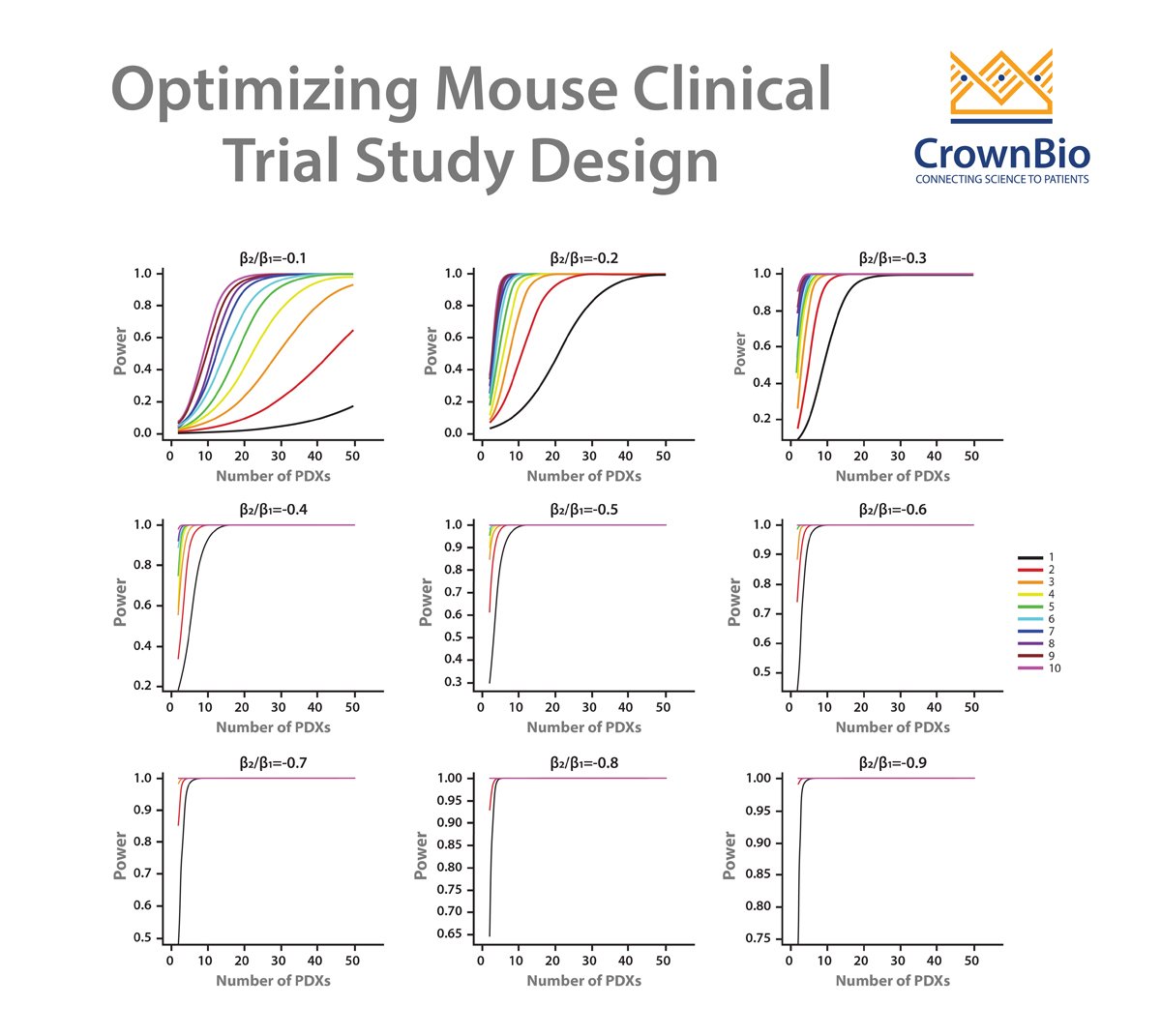
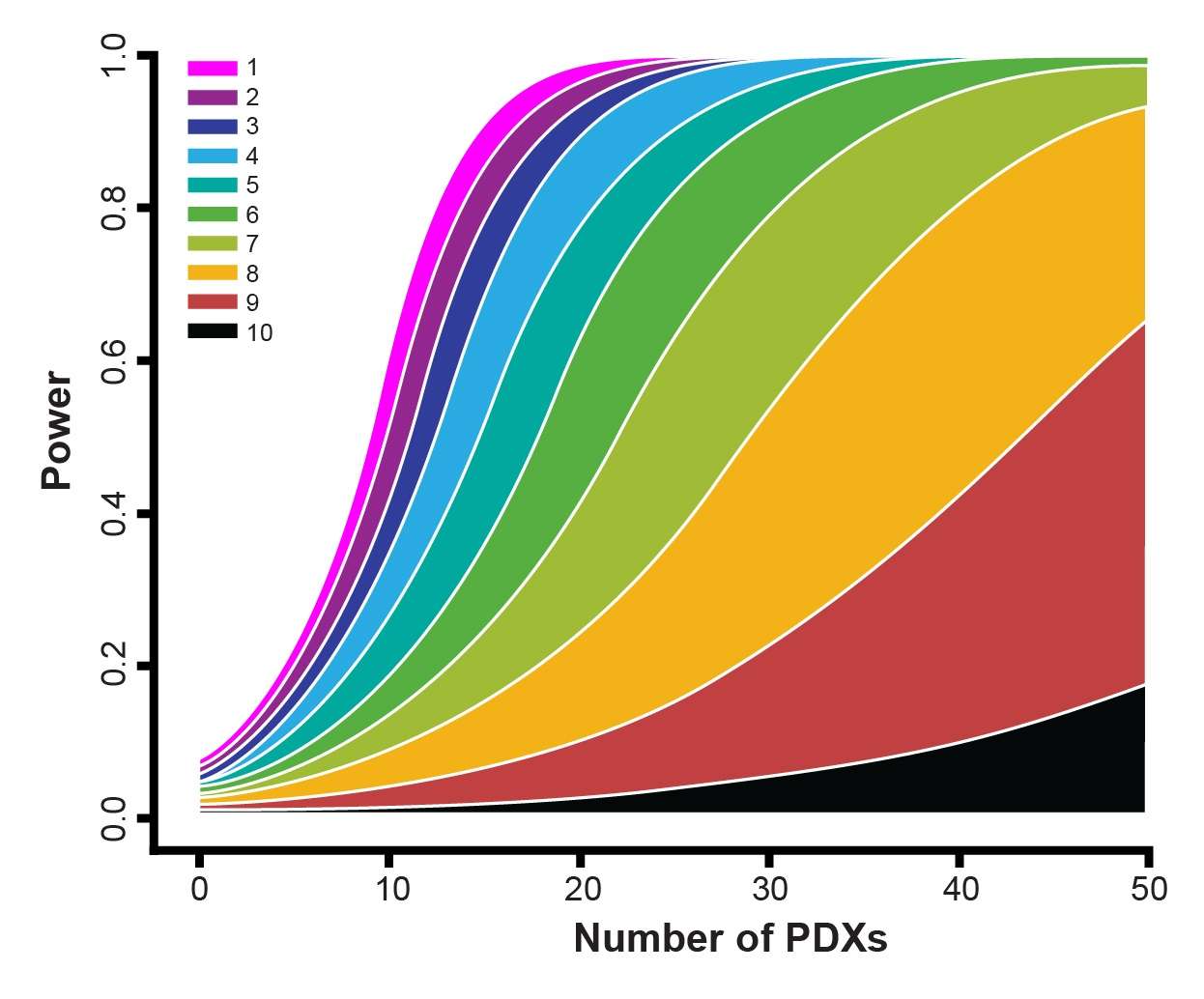
- Optimizing study design including how many PDX and mouse models should be used
- Quantifying drug efficacy
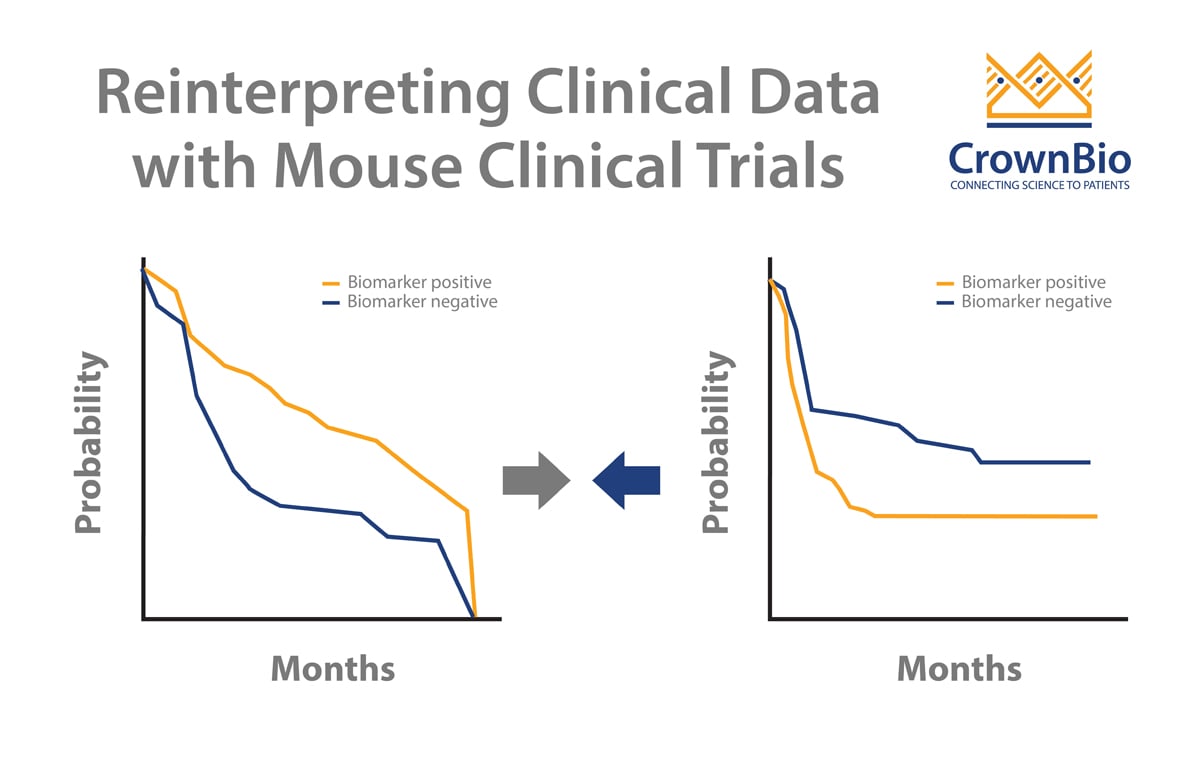
- Correctly evaluating drug effect by moving beyond conventional survival analyses
- Discovery and validation of biomarkers
- Reinterpreting clinical trial results
Conclusion: Advanced Mouse Clinical Trial Analysis Leads to Better Outcomes
Mouse clinical trials better represent human clinical trials in the drug discovery process, but it’s becoming clear that analysis methods can’t just carry over from the clinic to MCTs. Endpoint-based analyses don’t fully use the wealth of data generated from the clustered longitudinal studies.
To take full advantage of the power of MCTs new statistical frameworks are needed, such as linear mixed models, which can model inter- and intra-tumor heterogeneity of growth rate and drug response. These advanced analyses allow the full potential of MCTs to be explored, and to maximize the drug development programs they are part of.