 Mouse Clinical Trials (MCT) using patient-derived xenograft (PDX) models are a key preclinical tool for predicting drug effectiveness, targeting responder populations, and identifying biomarkers before you enter the clinic. MCTs are designed to enhance preclinical predictivity and help to reduce the current 95% attrition rate of oncology agents in clinical trials.
Mouse Clinical Trials (MCT) using patient-derived xenograft (PDX) models are a key preclinical tool for predicting drug effectiveness, targeting responder populations, and identifying biomarkers before you enter the clinic. MCTs are designed to enhance preclinical predictivity and help to reduce the current 95% attrition rate of oncology agents in clinical trials.
One key element of MCTs is the optimization of their study design, to ensure that the correct number of models and mice are used to accurately quantify and evaluate drug efficacy as well as leverage biomarker discovery.
Our recent webinar covered the power behind MCT study design, and this post reviews the top 10 questions raised during the event, answered by Dr Sheng Guo, Senior Director of Bioinformatics.
Question 1: Novartis' mRECIST does not take into account any control. In the simulation curves you showed, I presume you need a control arm. Which statistic do you use to quantify the response?
Answer 1: We used a control arm to perform simulations to obtain the power curves. The ratio of tumor growth rate between two arms are used to quantify the response.
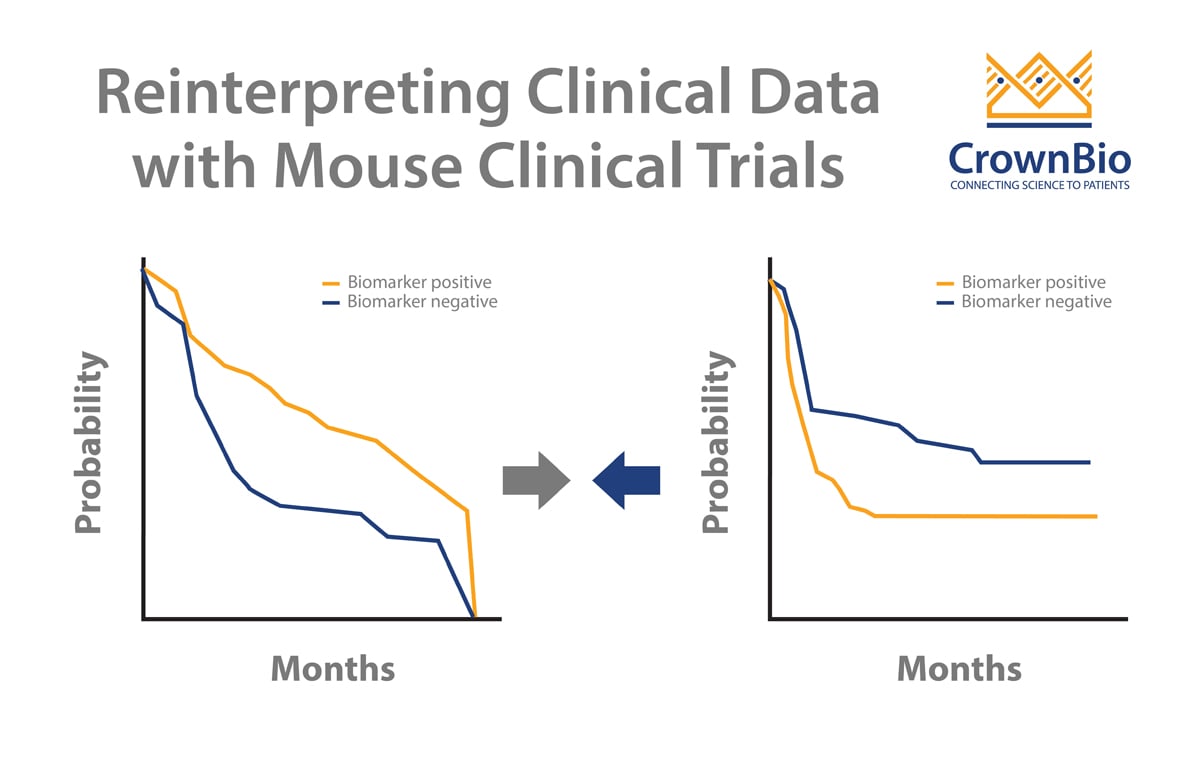
Question 2: To me the most important question is regarding the correlation between preclinical antitumor efficacy and clinical benefit. Do you have correlations with preclinical data from a PDX derived from a patient that went on to receive the same drug? How much preclinical effect should we want to see to expect a clinical benefit?
Answer 2: The correlation between PDX preclinical studies and clinical studies has been widely studied, and good agreement is observed in multiple studies, some can be found in this review paper:
Question 3: In PDX model experiments, which immunodeficient mouse is better? Nude, NOD Scid, or NSG™?
Answer 3: Mouse strains affect tumor take rate, e.g. the NSG mouse has a higher take rate. For established PDX models, the difference on drug evaluation is usually not considered.
Question 4: How should you adjust treatment duration based on growth rate?
Answer 4: For PDX models with slow growth it is advisable to extend treatment duration, especially if survival analysis is to be performed.
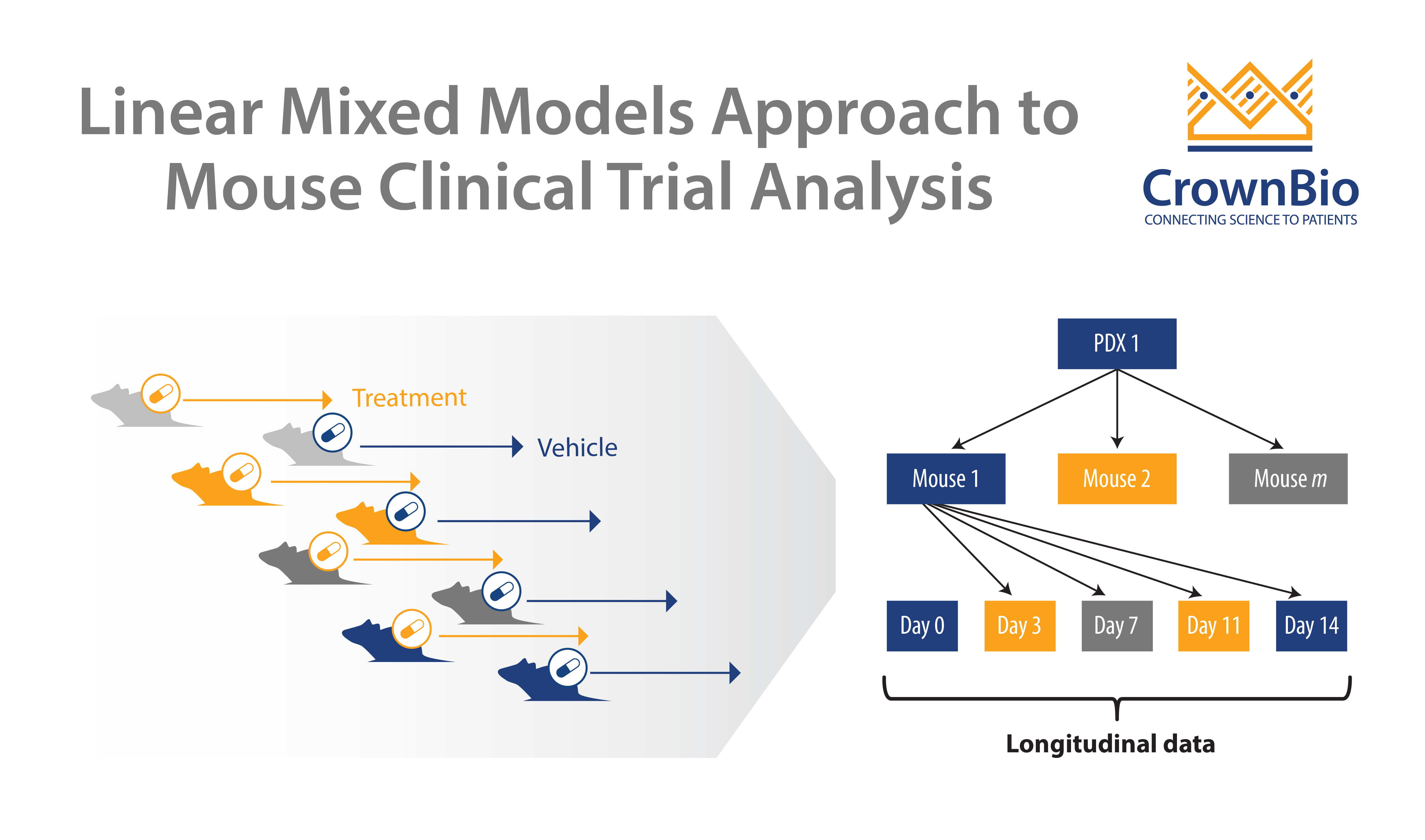
Question 5: In slide 35 of the webinar what is the statistical method used?
Answer 5: A linear mixed model (LMM) based approach.
Question 6: Can you comment on the 1x1x1 or 1:1 design?
Answer 6: This is the one of the questions that I have been asked the most, and I’ve touched upon it in places during the webinar, and I would like to comment on a few points here.
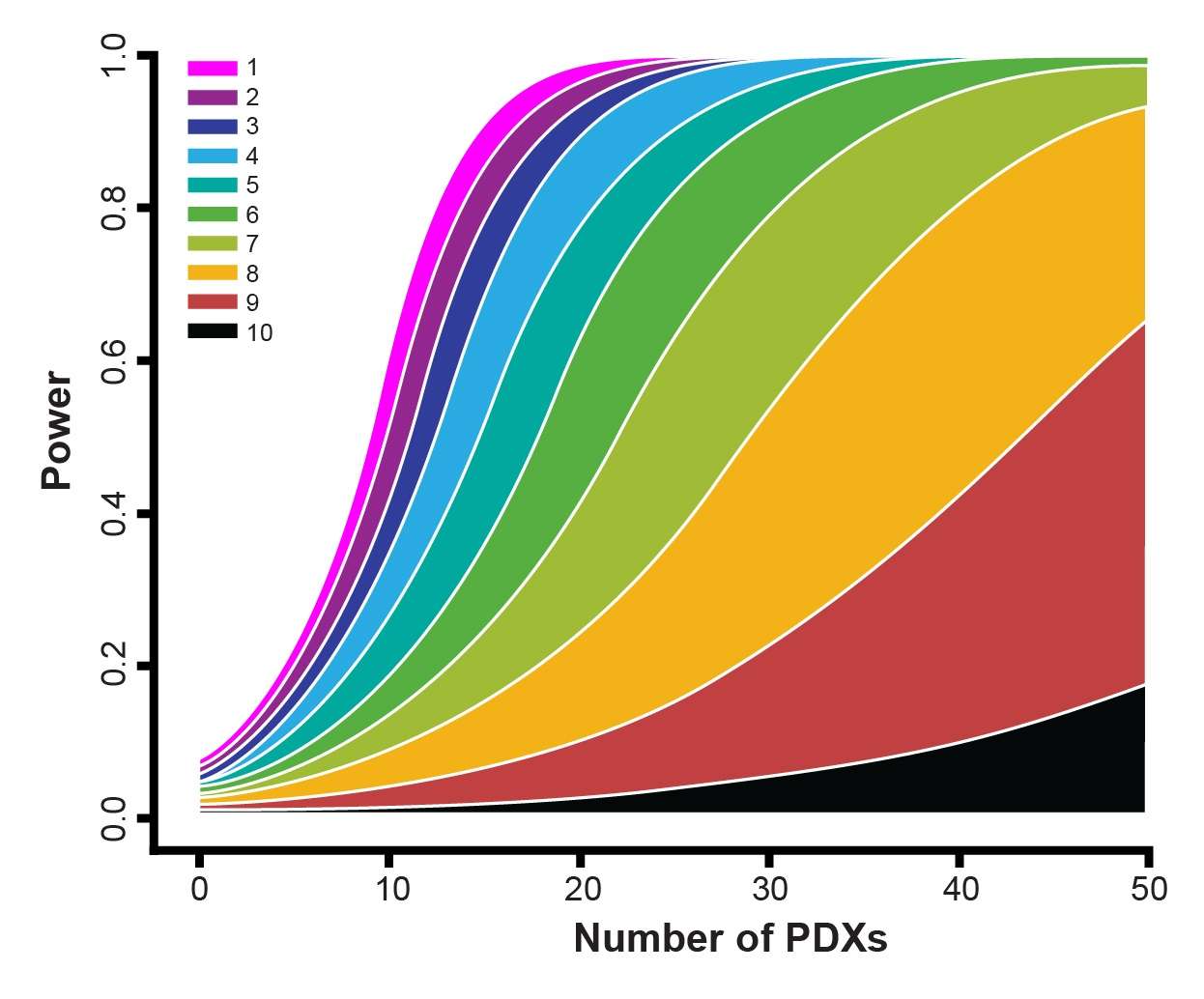
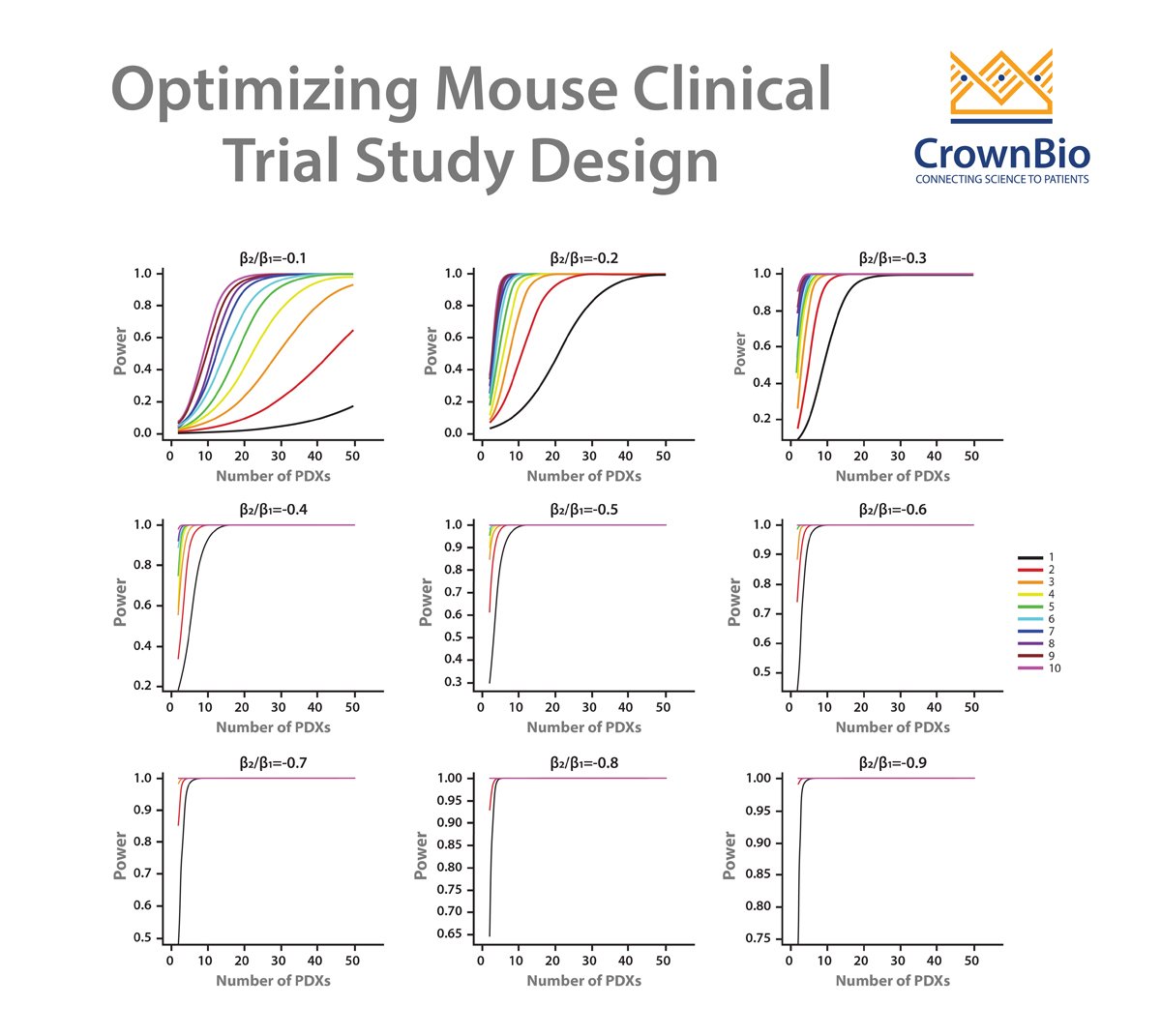
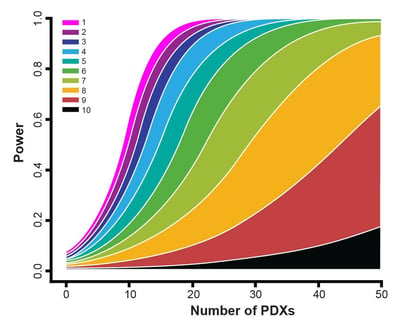
- The 1x1x1 or 1:1 design is actually a special case under our statistical frameworks (both the LMMs and the survival analysis) as you can see from the power curves within the webinar. Therefore, it can be dealt with in the same way as the other designs such as a 3:3 design.
- The 1:1 design is a good choice for general efficacy evaluation and biomarker discovery, provided that you have a respectful number of PDX. If you only have a handful of PDX, it is probably not ideal. The power curves can give more specific answers.
- From a statistical point of view, what really matters is not simply the number of PDX or the number of mice per PDX, but the total number of mice. Again, the total number of mice. If you check the power curves carefully, you will notice that the statistical power of the following 3 designs are close. A trial with 50 PDX, each with 3 mice in an arm, or a trial with 30 PDX, each with 5 mice in an arm, or a trial with 150 PDX, each with just 1 mouse in an arm. What do these trials have in common? 150 mice in an arm! From a biological point of view, it is good to have more PDX. So the 1:1 design is a top pick. Of course, this is not considering cost, because you would need to pay more for a mouse in the 1:1 design.
- The purpose of your trial also carries a big vote. If you are doing biomarker validation studies, then again, you need to get a relatively accurate measurement of efficacy for each PDX. The 1:1 design is therefore usually not optimal, especially when under circumstances such as your pool of candidate PDXs may be small, e.g. there are only a limited number of PDX carrying an IDH2 mutation. If you are doing biomarker validation and discovery at the same time, that is, you have some idea of your favorite biomarker, but you are unsure if it is the one, and want to check if there are other biomarkers as well, then, it is good to have a few mice per PDX. It is a balance. If you are doing a syngeneic immuno-oncology study, I would definitely not recommend the 1:1 design, as unlike PDX, very big efficacy differences are observed among mice of the same syngeneic model.
- Finally, I want to emphasize that the analysis of trial data is as important as trial design. Due to the relatively recent introduction of MCTs, and their resemblance to human clinical trials, many analytical methods for human trials were directly applied to MCT, with modifications sometimes included. Yet a distinct feature of an MCT is having mice of the same PDX in both arms, that is, self as control, and possibly with replicates. These are unseen in human trials, and should be explicitly modeled, that’s why we are advocating LMMs and more proper survival analysis, not just simple KM analysis and Cox proportional hazard models.
Question 7: Once you have decided which study design to move forward with, what is the best way to do model selection?
Answer 7: PDX models can be selected based on their genomic, pharmacological, and growth profiles, many of which are available within our PDX database: http://hubase.crownbio.com
Sometimes, more complex features are needed, such as MSI status, activation of certain pathways, or subtypes of a cancer. Our bioinformatics group frequently performs tailored research to identify suitable models.
Question 8: How are your PDX samples typically characterized prior to the study?
Answer 8: Many of our models, for example, have RNAseq data (for >1500 PDX models in our collection) and we are actively perform characterization and profiling on all our PDX models. For example, we used computational approaches to perform molecular pathology study of our PDX models, such they are correctly classified into cancers and cancer subtypes.
Question 9: On slide 24 in the webinar the case study you talk about using 42 PDX models. Is that a typical trial size?
Answer 9: A “typical” mouse trial can be large or small. Smaller MCTs usually involve 20-30 PDX, and the larger trials can have several hundred models.
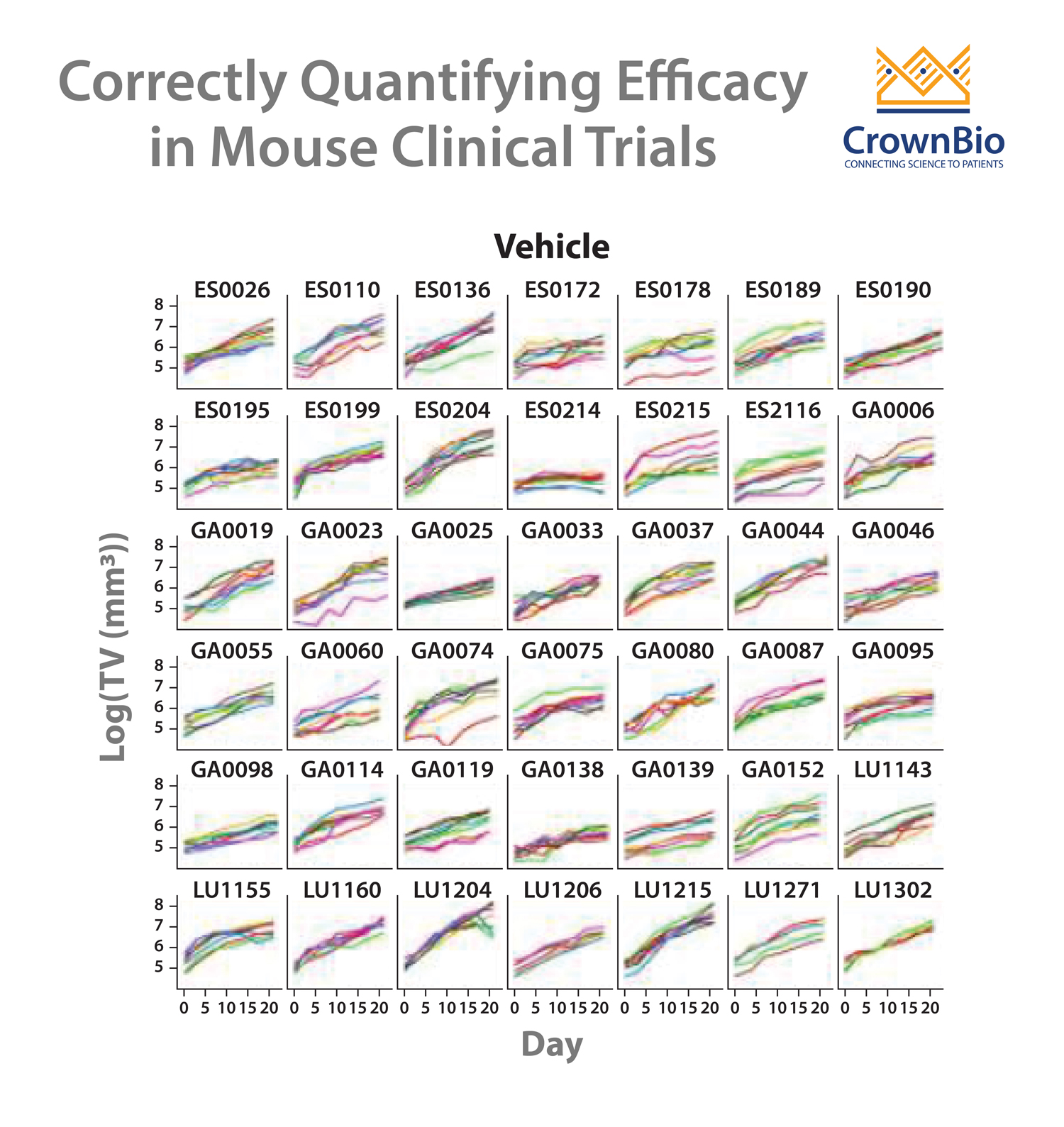
Question 10: What if the growth curve is not linear?
Answer 10: Based on our experience, at least 90% of the time, the growth curve, after log-transformation, is close to linear, or can be described by linear equation with quadratic terms. They might be better described by more complex equations, such as Gompertz equation. However, due to the simplicity and availability of powerful statistical machinery, we still prefer LMM. In the event when they cannot, we can still use simpler LMM to model the data still captures the matching and pairing structure of the trial.
If you have any further questions on the MCT study design webinar please contact us