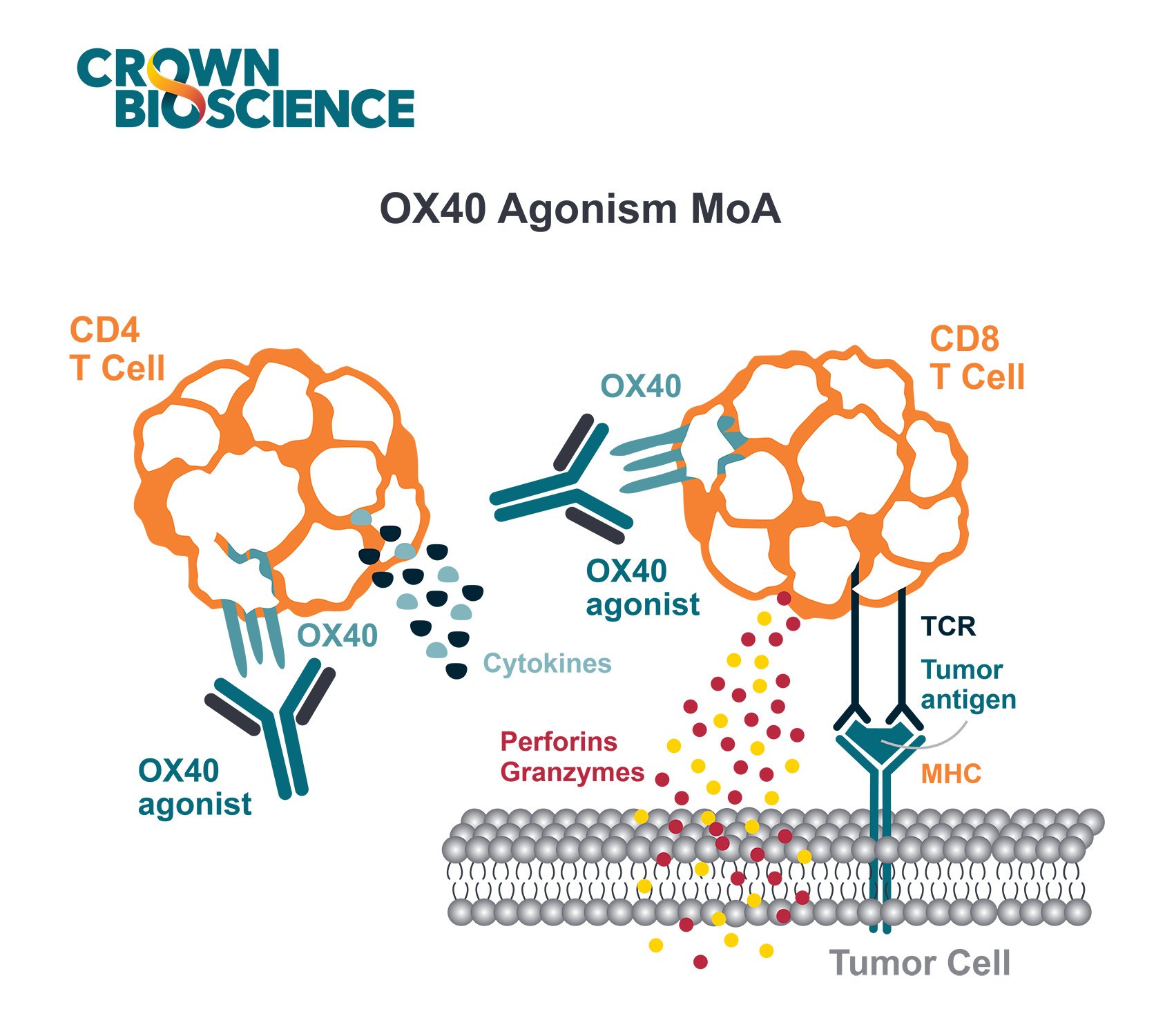
 Patient-derived xenograft (PDX) models are typically immunodeficient and inappropriate for most immuno-oncology (I/O) drug discovery. Humanized PDX models, however, have functional human immune systems for therapeutic modulation. This opens up new applications for these highly predictive models in the immuno-oncology research space.
Patient-derived xenograft (PDX) models are typically immunodeficient and inappropriate for most immuno-oncology (I/O) drug discovery. Humanized PDX models, however, have functional human immune systems for therapeutic modulation. This opens up new applications for these highly predictive models in the immuno-oncology research space.
Immunotherapy Assessment in PDX Cancer Models
Immuno-oncology therapies stimulate the immune system to act against cancer rather than attacking it directly. This means that I/O agent research and evaluation needs to take place within a functional immune system for the novel treatments to modulate.
With their close recapitulation of human disease, and ability to provide highly predictive data, PDX models are key tools in preclinical oncology research. By humanizing these models via CD34+ HSC for a long-term reconstitution in mice, PDX can support comparable applications in immuno-oncology research.
Benefits of Humanized PDX Models
Humanized PDX have four main advantages in immunotherapy drug development:
- Relevance to human-specific antibodies. This can be essential if no mouse surrogate exists or there is no cross reactivity.
- Relevance to both known and novel immuno-oncology targets. Using humanized PDX as compared with drug target-humanized models (e.g. human PD-1 within a murine immune system) lets you evaluate multiple I/O targets while more accurately modelling the diversity of human lymphocyte targets.
- The ability to perform humanized Mouse Clinical Trials (MCT) by better capturing the diversity of immune systems and tumors within a population.
- Supports study of combination regimens, such as I/O + targeted agents (accessing the full diversity of cancer genetics from a PDX collection e.g. PD-1/EGFR, CTLA-4/BRAF) or for dual immunotherapies.
Humanized PDX Mouse Clinical Trials (MCT)
Response to immunotherapy depends on the immunological state of the host immune system and the genetic/phenotypic traits of the cancer. Translatable, preclinical immuno-oncology research must mimic this variation to provide predictive data and help guide biomarker discovery for patient stratification.
Humanized PDX models are used in Mouse Clinical Trials (MCT) to reproduce and overcome this variability. n=1 checkerboard designs test multiple PDX models, across multiple stem cell donors, to account for donor-to-donor variability. This recapitulates a clinical trial with human patients, as each model/donor is a unique combination.
Correlation has been shown between humanized MCTs and clinical trials, validating their use as key preclinical I/O tools.
Models can be selected based on genetic screening data, as with PD-L1 expression for anti-PD-1 evaluation. Once selected, a small panel of models is then arrayed across a selection of immune donors. For example, seven PDX combined with five immune donors results in thirty-five separate patient “avatars” for analysis.
Modelling Variations in Patient Response
Endpoints such as tumor growth inhibition, tumor-infiltrating lymphocyte analysis, and histology can show a mix of responders and non-responders, often with no consistent PDX responder across all donors or immune systems. This has spurred interest in developing a greater understanding of what patient and tumor characteristics control this response.
Downstream immunophenotypic analysis can start to answer these questions by comparing the characteristics of responders and non-responders. These analyses can cover general tumor infiltrating immune cell populations, as well as more specific marker assessment to deduce which cell types may promote or inhibit response.
Humanization of Conventional PDX to Study Memory Response
Going back a step, humanization is needed to observe the in vivo efficacy of certain immuno-oncology agents, such as PD-1 treatment.
With faster growth and lack of lag time, humanized conventional cell line-derived xenografts are ideal for these proof-of-concept studies. For example, looking at anti-PD-1 effects on a triple negative breast cancer conventional xenograft, tumor growth inhibition will only be observed in a humanized setting.
These humanized xenografts can then be rechallenged with the same treatment. If the rechallenge is successfully rejected in the previously-treated cohorts, this shows the memory response characteristic of a “true” immune response.
Humanized PDX Expand Immuno-Oncology Research Capabilities
Humanized PDX models are a vital resource in immuno-oncology drug development, enabling researchers to model interactions between drugs, cancer, and the immune system with the heterogeneity of a true clinical environment.