 In this introduction to preclinical imaging, we explore optical imaging technologies, such as bioluminescent and fluorescent imaging, and their applications to in vivo preclinical oncology models and cancer drug development.
In this introduction to preclinical imaging, we explore optical imaging technologies, such as bioluminescent and fluorescent imaging, and their applications to in vivo preclinical oncology models and cancer drug development.
Preclinical Imaging Technologies
“Preclinical imaging” encompasses a range of technologies which can be used separately or in combination. These include both anatomic — X-ray, computed tomography (CT), magnetic resonance imaging (MRI) — and molecular modalities, such as positron emission tomography (PET) and optical imaging.
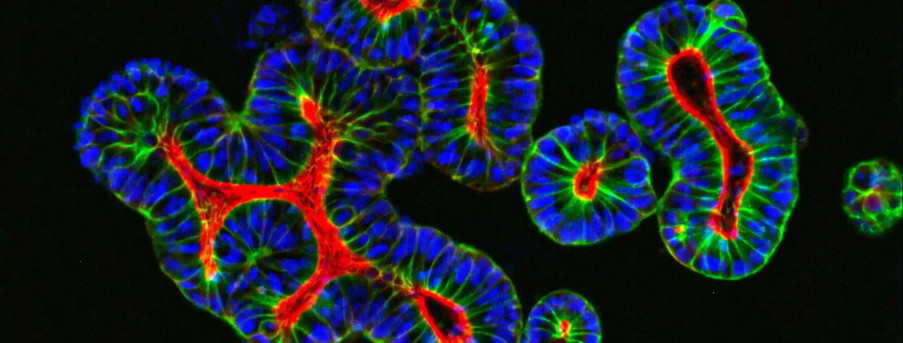
Of these technologies, optical imaging has become increasingly popular. Here, we use fluorescent or bioluminescent tissues, or probes, which are detected non-invasively in living animals. The popularity of optical imaging is due to its low cost, ease of data interpretation, and the flexibility of probes which are used to visualize many aspects of our preclinical investigations.
Preclinical Optical Imaging in Cancer Models
Optical imaging has found a niche in oncology research. In preclinical oncology, conventional subcutaneous tumor models are well-established, remaining a consistent element in the development of new drugs. Simple subcutaneous tumors are easy to implant and measure, allowing straightforward assessment of disease development.
With recent developments in the generation of patient-derived (PDX) models, syngeneic models, and genetically-modified animals developing spontaneous tumors, today’s researcher has a wide range of subcutaneous models to choose from.
However, when we use more sophisticated orthotopic, systemic, and metastatic models, the assessment of disease burden can be more challenging. These models are useful as they more accurately mimic patient-relevant lesions than conventional models. Growing at the clinically-relevant site, they tend to develop more representative tumor vasculature and a closer recapitulation of tumor microenvironment. This all leads to a more accurate prediction of response to therapy.
As these models often can’t be palpated, or grow at distant sites from the initial implantation, it’s difficult to determine individual animal disease burden at the start of a study. This requires larger group sizes to account for variability, and tumor burden is often only measured using terminal end points.
Optical Imaging: Understand Disease Mechanisms with Real Time Monitoring
This is where optical imaging comes into its own. Imaging enables us to track disease development and spread in individual animals throughout the course of a study. Presence of disease in animals can be measured before treatment starts and at a variety of timepoints during disease development.
Monitoring disease during drug treatment also effectively allows each animal to become its own control.
In addition, imaging also allows the visualization of appropriately-labelled entities, such as therapeutics, in real-time, in vivo. When used with other complementary imaging technologies, such as X-Ray, CT, or MRI, we can coregister anatomical data with biological data to gain a deeper understanding of the mechanisms at play.
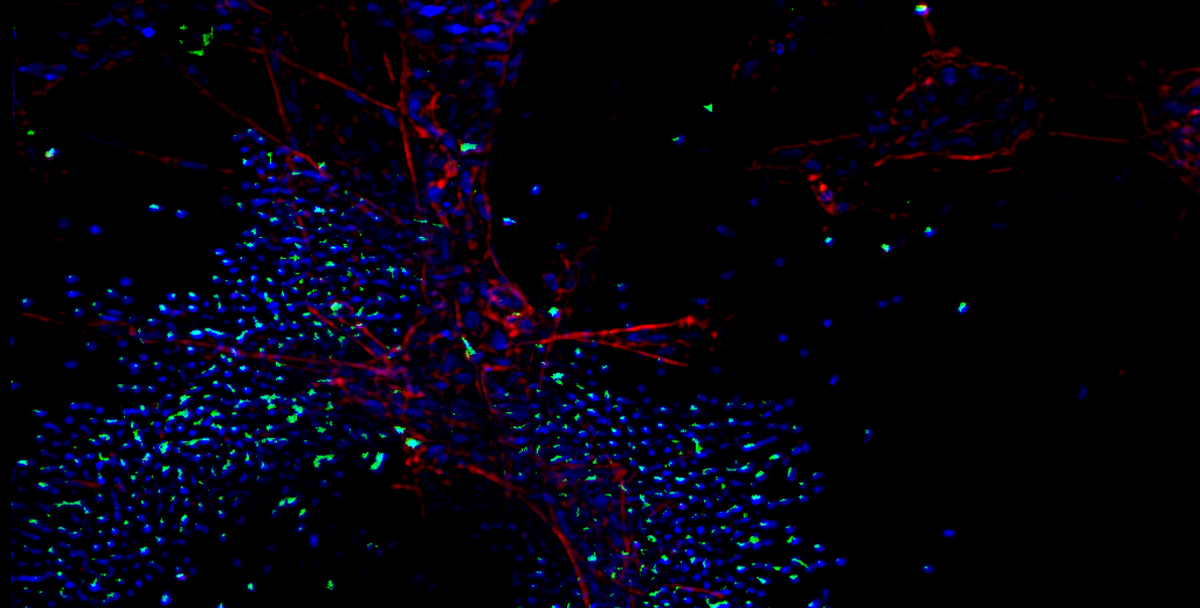
Bioluminescence and Fluorescence Imaging
Optical systems use both bioluminescence and fluorescence for imaging.
Bioluminescent Imaging
Bioluminescence is a biochemical reaction – an enzyme is exposed to its substrate and emits light. For optical imaging, firefly luciferase is most often used with its substrate luciferin, although several other luciferases are also available.
The creation of a bioluminescent cell line is a relatively straightforward process using stable lentiviral transduction. This negates the need for clonal selection, as would be required with transfection. Luciferase positive cells are either made bespoke in-house or bought from a range of commercial suppliers. In-house generation is followed up with STR profiling to ensure a DNA profile match with the parental line, an overall process that can take as little as five weeks.
Luciferase-expressing cells are then inoculated into experimental animals. In a typical experiment, the subject is injected with luciferin immediately prior to imaging. As the luciferin reaches cells expressing luciferase, they emit light, which is detected and correlated to disease burden.
Optical imaging has allowed the development of highly elegant cancer models opening a wealth of possibilities for research across:
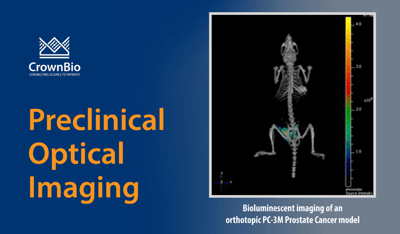
- Tumors directly implanted into deep lying organs such as the brain, prostate, lung, and liver.
- Metastatic models of breast cancer and the lymphatic system.
- Models of malignant bone disease.
- Models of hematological disease such as leukemia.
Fluorescent Imaging
Where bioluminescent imaging is not possible or appropriate, fluorescent imaging can be used. A wide number of different fluorophores are commercially available.
In vivo optical imaging using visible light is challenging, as components of animal tissue, such as hemoglobin, absorb and scatter incident excitation light of most visible wavelengths. This limits light penetration, resulting in low-fluorescence emission signals that are difficult to detect consistently.
A number of fluorophores have been developed with emission wavelengths over 680nm, in the near infra-red portion of the spectrum. These fluorophores are most suitable for imaging in vivo as tissue is effectively transparent at these wavelengths. Fluorophores can be used to label a wide range of entities, including; small molecule drugs, antibodies, or T cells, and give us the power to visualize a range of biological events happening in real time in a live animal.
Imaging Preclinical Models in Cancer Drug Development
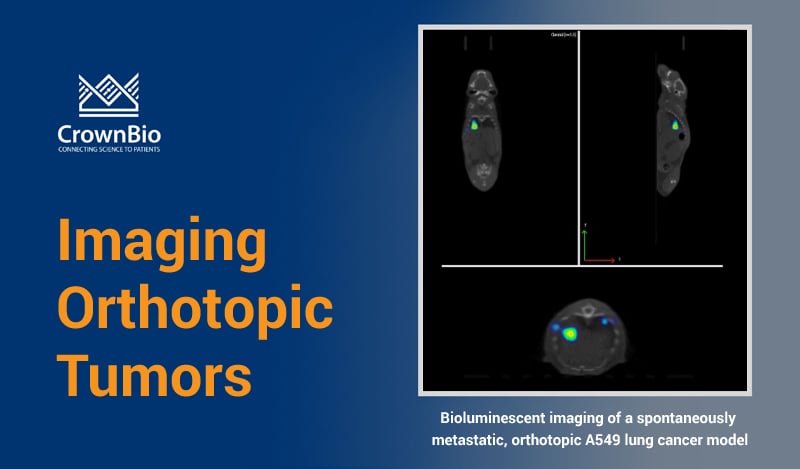
Many bioluminescent preclinical models have been developed for cancer drug discovery. These include bioluminescent orthotopic and metastatic cell line-derived xenograft models, as well as bioluminescent syngeneic models for immunotherapy development. These are combined with highly-sensitive detection technologies (e.g. IVIS Illumina III in vivo imaging system and the IVIS® Spectrum CT) to monitor cancer progression.
Imaging preclinical cancer models provides valuable disease information at different cancer stages. This includes orthotopic models to mimic primary lesions, and the imaging of both spontaneous and experimental metastases to replicate progression to late stage disease.
Optical Imaging of Molecular Processes
As well as allowing us to monitor tumor growth and metastasis in cancer models, optical imaging confers the ability to study a variety of molecular processes across a range of disease types, for example:
- Infection and inflammation.
- Distribution of labelled immune cells in disease bearing animals.
- Changes in microenvironment of tumors, development of necrosis, vasculature formation.
- Visualization and quantification of disease biomarkers.
- Imaging of reporter genes.
- Biodistribution of labelled drugs, therapeutic antibodies, ADCs, nanoparticles.
Imaging data can also aid in the optimization of dose and delivery strategies, finessing in vivo studies and ultimately improving translation from the lab to the clinic. Combinations of different luciferases and fluorophores allows us to answer several different questions in the same animal in the same experiment.
Summary
Optical imaging is a fast, sensitive, reliable, and cost-effective method to elucidate a wide range of biological questions:
- A range of bioluminescent and fluorescent markers allows the study of multiple biological questions in the same experiment.
- Allow us to follow orthotopic, metastatic, and systemic disease in real time in live animals, with the ability to assess disease burden throughout the duration of the study.
- Visualization of biodistribution of therapeutic entities such as antibodies, ADCs, nanoparticles, and small molecules in vivo.
- Can elucidate pharmacodynamic effects, and microenvironmental factors such as necrosis.
- Can be utilized in a wide range of disease models including cancer, infection, and inflammation.
- Reduces the number of mice needed per study with longitudinal follow up of each animal at several time points.
- Can be used in combination with other imaging modalities to provide anatomical reference points alongside biological data.