 Explore why glucocorticoid-induced TNFR-related protein (GITR or CD357) agonists are becoming increasingly important as alternative immunotherapy candidates for combination therapies.
Explore why glucocorticoid-induced TNFR-related protein (GITR or CD357) agonists are becoming increasingly important as alternative immunotherapy candidates for combination therapies.
What is GITR?
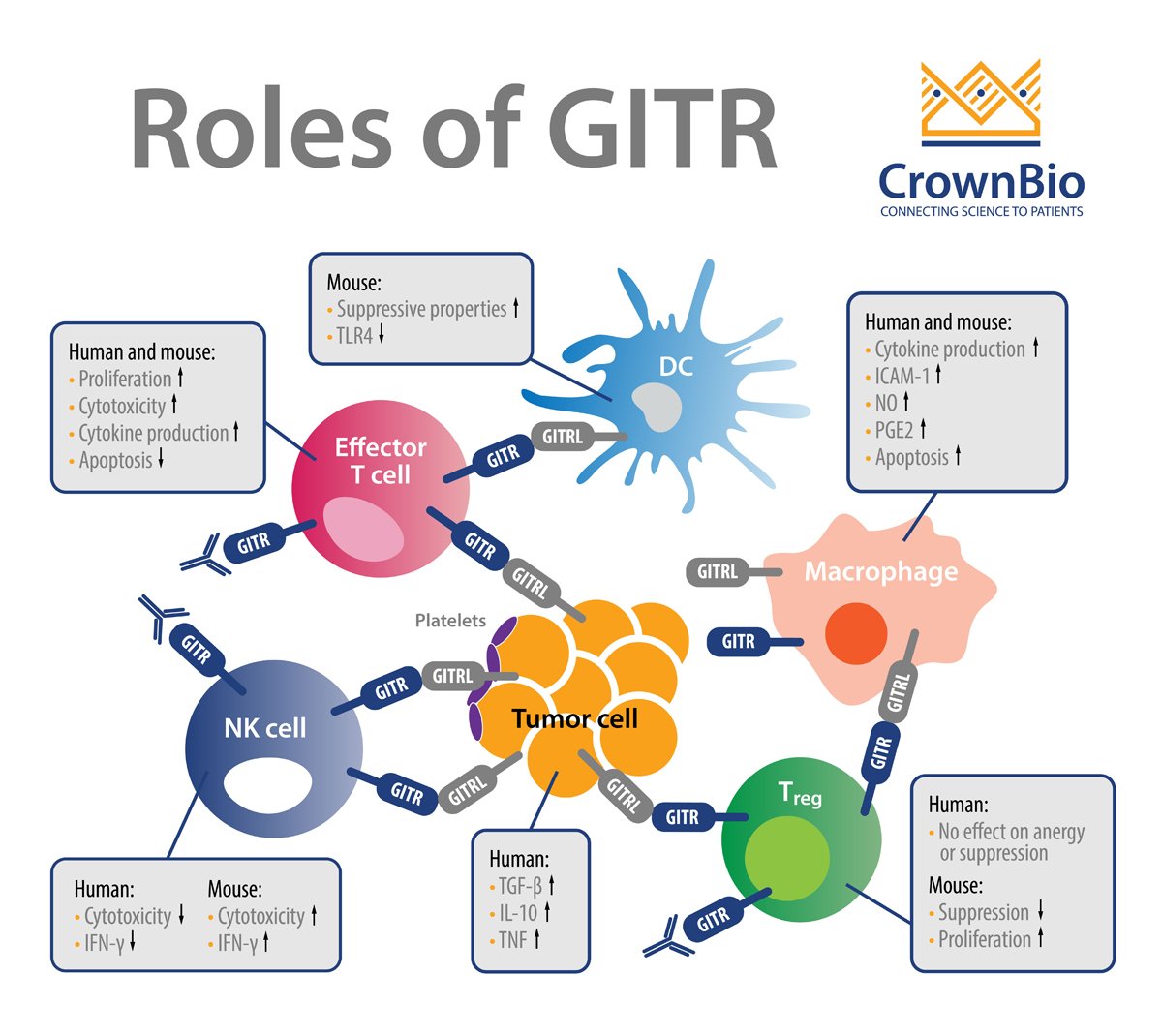
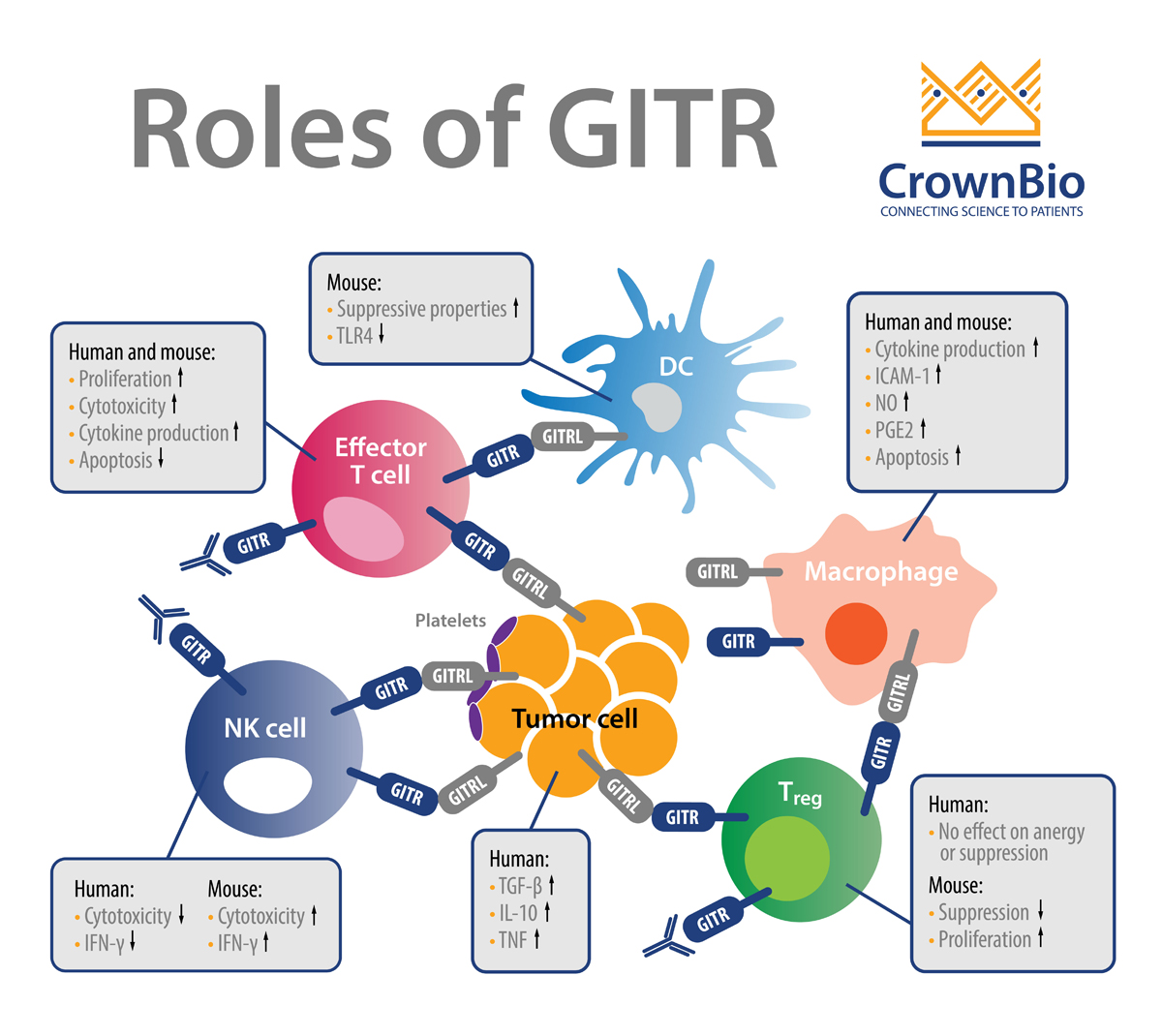
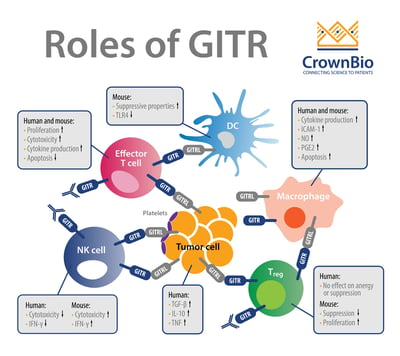
GITR is a well-characterized costimulatory receptor that is a member of the tumor necrosis factor receptor superfamily (TNFRSF). GITR is expressed in hemopoietic and non-hemopoietic cell lineages, e.g. natural killer (NK) cells, B cells, macrophages, dendritic cells, epidermal keratinocytes, and endothelial cells. Activation of GITR is by its ligand GITRL, which is also expressed on many cell types.
GITR’s Role in Immune Function
GITR is a surface molecule expressed on activated CD4 and CD8 T cells and FoxP3+ Treg cells. GITR activation regulates cell survival through MAPK cascades (ERK, JNK, p38 MAPK), and leads to upregulation of Bcl-xL and activation of NF-κB pathways (canonical and non-canonical). GITR’s signaling synergizes with T cell receptor (TCR) signaling and results in a cascade of events in T cells that:
- Enhances their proliferation
- Increases IL-2, IFN-γ, and CD25 expression
- Enhances effector functions
- Inhibits activation-induced cell death/apoptosis
- Increases survival and frequency of memory T cells
GITR activation on Tregs is thought to play a major role in the antitumor effects of GITR agonists and GITR ligand therapeutics. Recently, a broader GITR impact on other effector T cells has emerged where long-term GITR activation seems to enhance Treg activity and expand their numbers.
Notably, in tumor immune cell infiltrates, there is higher expression of GITR in Tregs than in effector T cells. It’s also not surprising that, similar to other immune checkpoints, shedding of GITR has been reported.
GITRL is also expressed on various immune cell types, including at high levels in antigen presenting cells, dendritic cells, B cells, and macrophages, and GITRL’s levels have been shown to be induced by IFN-β and LPS.
Overall, in the context of immune function, activation of GITR contributes to maximal CD8+ T cell responses against tumors and pathogens by promoting effector T cell function while inhibiting regulatory T cell (Treg) function.
Over recent years, GITR has been recognized as an important immune checkpoint pathway and has gained significant attention by drug developers who have pursued both preclinical and clinical studies with GITR agonists.
Why Does GITR Matter for Immunotherapy?
Since some patients do not respond to current checkpoint blockade immunotherapies, significant research activity has been directed towards developing novel approaches for targeting alternative immune pathways. The overall goal is to extend the benefits of immunotherapy to a greater number of patients.
GITR is an example of a costimulatory immune checkpoint pathway that has gained significant traction over recent years. For instance, monoclonal antibodies (mAbs) targeting GITR have shown great promise because they enhance CD8+ and CD4+ effector T cell activity while inhibiting/depleting Tregs. GITR also has the footprint of a classic immune checkpoint where agonistic GITR antibodies worsen the immunopathology associated with autoimmunity (e.g. in autoimmune gastritis, collagen-induced arthritis, experimental autoimmune encephalomyelitis, diabetes).
Many preclinical studies have successfully used syngeneic mouse models with a variety of cell lines (e.g. B16-F10 melanoma, CT26 colon carcinoma, MC38 colon adenocarcinoma, and TC1 HPV/cervical cancer model) to show that GITR agonists such as GITR Ligand Fusion Protein (GITRL-FP) induce antitumor activity and enhance the tumor antigen–specific CD8 T cell response. Agonistic antibodies have been shown to inhibit tumor growth as a single agent or in combination with other drugs.
A study has also reported that intra-tumoral injection of the anti-GITR agonistic antibody “DTA-1” showed fewer auto-reactive T cells in the spleen as compared to systemic delivery of the antibody. The authors suggest that intra-tumoral delivery may be a more effective approach to induce an antitumor immunity as compared to systemic delivery of GITR agonistic antibodies.
Additional technologies will certainly continue to help advance GITR agonists, such as functional in vitro screens using reversible T cell dysfunction assays and more human relevant preclinical models, such as PDXs and patient-derived tumor organoids (PDO) populated with characterized immune cells.
Clinical Progress with GITR Agonists
Significant clinical progress has been made with investigational GITR agonists, which generally seem to have acceptable safety profiles. For example, in one recent study MK-4166 (an anti-GITR mAb) was found to be well tolerated, and when combined with pembrolizumab (Keytruda®) an objective response rate of 69% (9/13) was reported. Additional ongoing clinical trials testing GITR agonists in combination with other drugs are also showing great promise, albeit it is important to mention that as a monotherapy we are not aware of any GITR agonists showing clinical efficacy.
A recent report of GITR expression across multiple tumor types indicates that some cancer types express GITR on malignant cells including 6% of breast cancers, 5.7% of bladder cancers, 4.5% of primary (but not metastatic) melanomas, and 3.2% of ovarian cancers. The investigators suggest that GITR expression on these tumors may signal towards a new tumor immune evasion strategy that should be explored in additional studies.
Below is a list of investigational GITR agonists (with corresponding clinical trial number and link) that are being tested in combination I/O regimens:
- TRX-518: NCT02253992, NCT01239134, NCT02628574, NCT03861403
- BMS-986156: NCT02598960, NCT04021043
- AMG228: NCT02437916
- MEDI1873: NCT02583165
- MK-4166: NCT02132754, NCT03707457
- MK-1248: NCT02553499
- INCAGN1876: NCT04225039, NCT02697591, NCT03277352, NCT03126110
- GWN323: NCT02740270
- ASP1951: NCT03799003
Conclusion
GITR signaling is a strong immune modulator and this pathway is actively being studied in preclinical tumor models and human patients to enhance antitumor responses.
GITR agonists have shown very promising results in tumor growth inhibition, extended survival, and depletion of tumor infiltrating Tregs which was thought to be the primary mechanism of action. However, more recent studies indicate that GITR signaling impacts multiple immune cell subsets including CD8+/CD4+ T cells, antigen presenting cells, and NK cells. In addition, there is emerging data on bidirectional signaling of GITR engagement which likely implicates even more complex pathways.
To fully exploit preclinical studies using GITR agonists, research programs should include multiple in vitro and animal models to best characterize GITR agonists whether anti-GITR agonistic antibodies, GITRL, or GITRL-FP.