 Preclinical oncology drug development needs new tools and approaches. Current methods result in 95% attrition rates for cancer agents in clinical trials due to a lack of efficacy, even though the potential drugs looks highly promising in preclinical testing.
Preclinical oncology drug development needs new tools and approaches. Current methods result in 95% attrition rates for cancer agents in clinical trials due to a lack of efficacy, even though the potential drugs looks highly promising in preclinical testing.
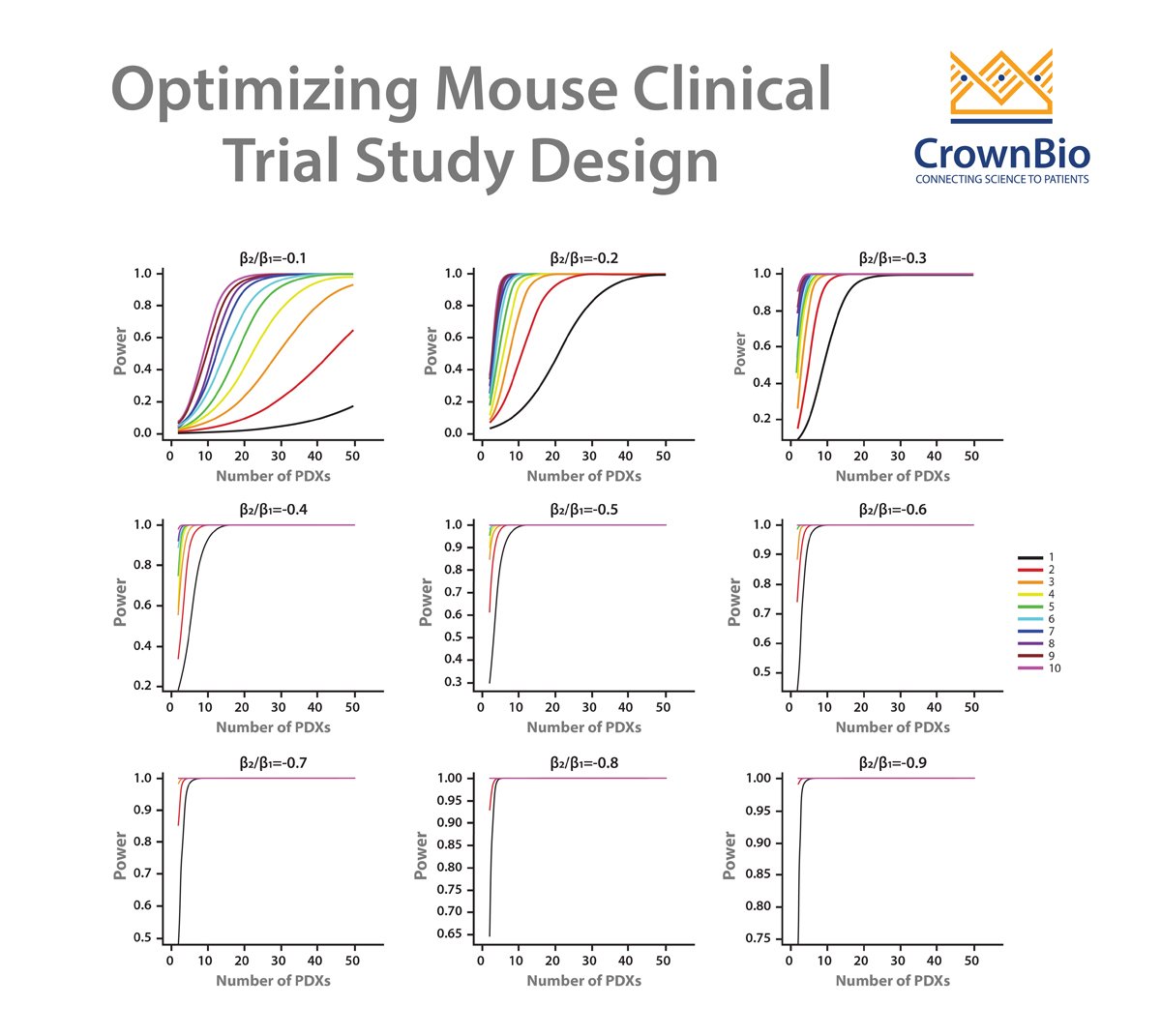
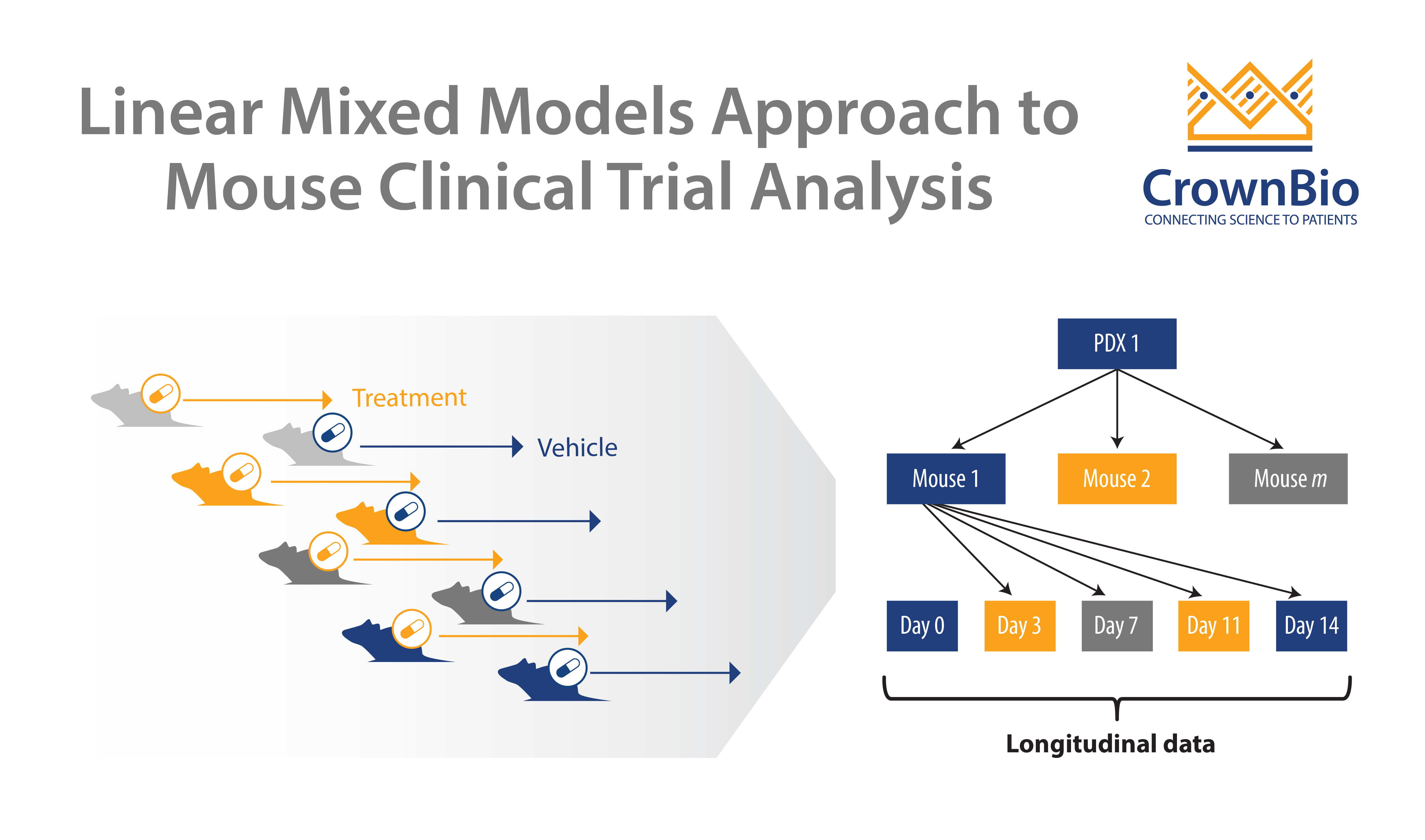
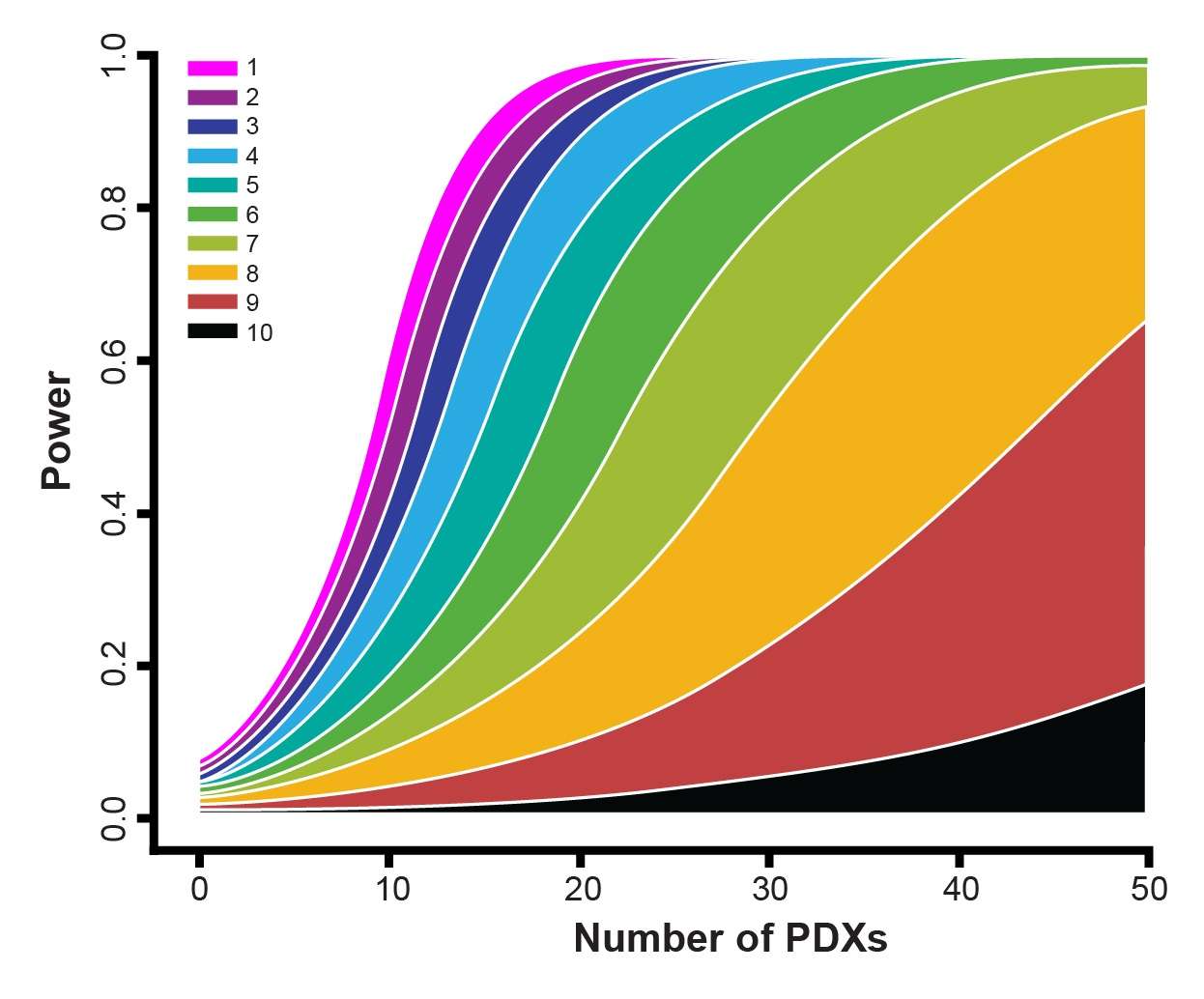
Clearly, there is a challenge that needs to be overcome. Precision and Predictive Medicine are clearly part of the solution, but in the short term new methods are needed to provide more predictive outcomes in the preclinical space. One such method is Mouse Clinical Trials, also known as MCTs. These studies use cohorts of patient-derived xenograft (PDX) models to represent a clinical study population, within a randomized, controlled, and statistically powered setting, to provide highly predictive data.
This blog post takes a look at the Top 5 reasons to try out MCTs in an oncology drug development program.
1. Mouse Clinical Trials More Closely Represent the Human Clinical Trial Situation
In human clinical trials a group of patients (each with a different background and heterogeneous disease) are treated with the same novel agent/treatment regimen – one person receives one treatment. Current preclinical methods are different – they use a small number of xenograft models with a large subject number per arm.
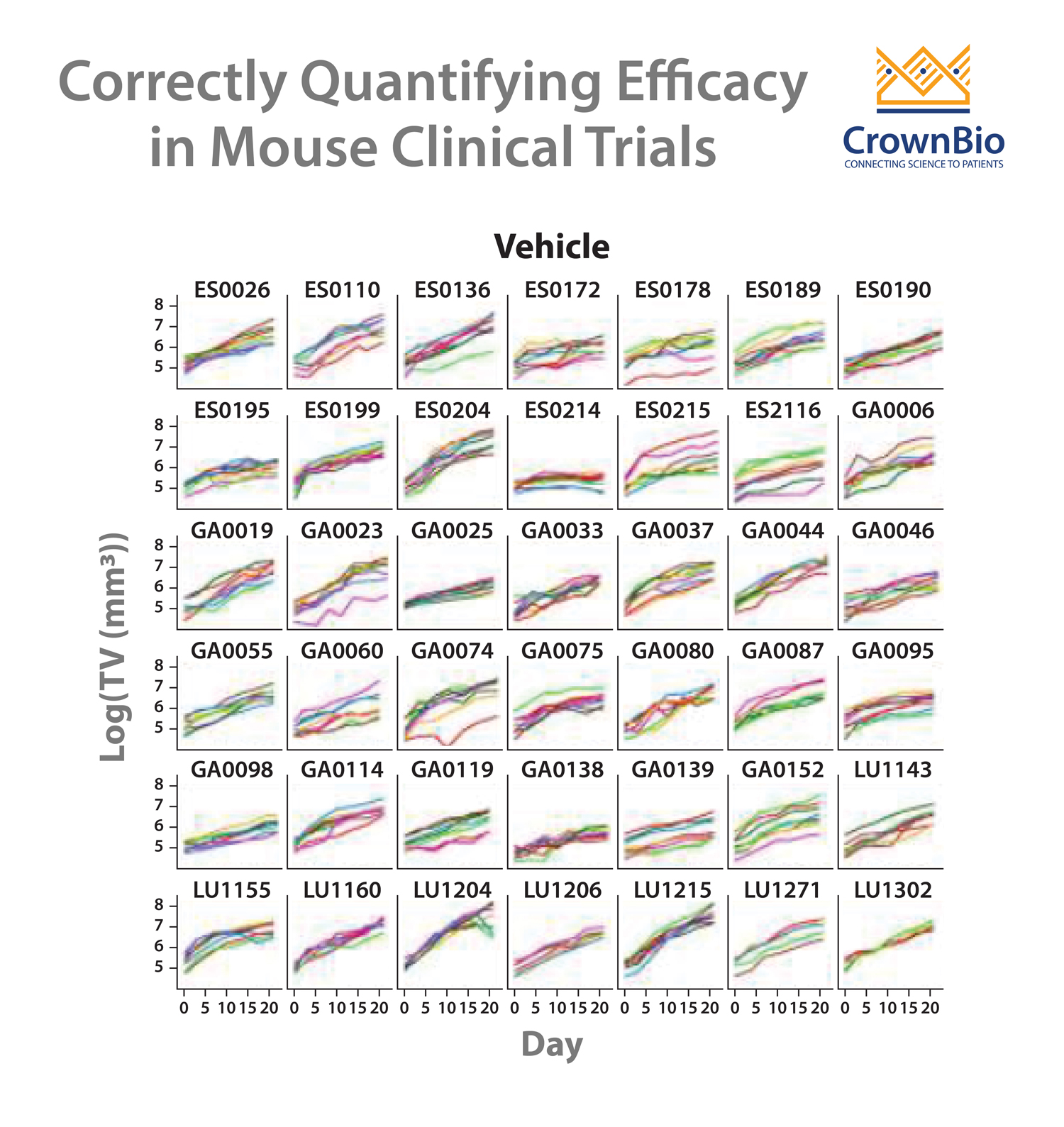
Therefore, the clinical and preclinical strategies don’t match up. MCTs upend the traditional preclinical paradigm and more closely match the clinical setting – using a large number of models (representing the large group of patients) in a small number of animals (representing the individual patients in the study).
This method provides a better perspective of patient-to-patient heterogeneity and the resulting data can be leveraged for biomarker discovery.
2. MCTs Use the Most Predictive Preclinical Mouse Models Available
Traditional oncology drug development has relied on cell line derived xenograft models. While these models are useful for early stage drug development, they tend to have drifted from original disease due to long term tissue culture and don’t really capture the true heterogeneity of the human clinical oncology population.
A more predictive alternative which is used in MCTs are patient-derived xenograft models, PDX are recognized as the most predictive preclinical model. They are created from patient tumors which are implanted directly in mouse models and are never manipulated to grow in vitro. A lack of selection pressure and the recapitulation of diverse patient genotypes produces a more translational model, more reflective of patient heterogeneity and response to treatment, ideal for truly understanding preclinical efficacy and providing predictive data before clinical trials.
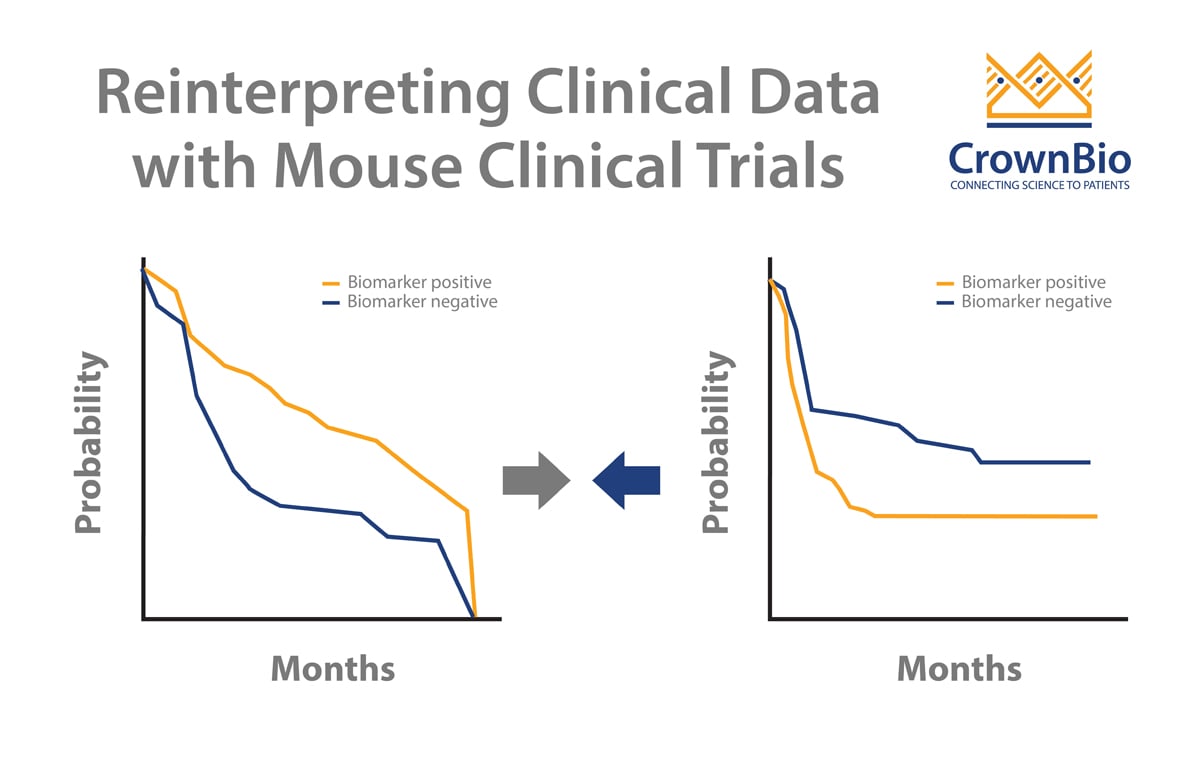
3. PDX Mouse Clinical Trials Enable You to Discover Who Will Respond to Your Agent Before You Enter the Clinic
Using PDX models in the MCT framework provides highly predictive data on exactly which models (and in turn patients) will respond to your agent, and the reasons why. PDX models are extensively characterized allowing response and genetic background to be linked. Therefore responder and non-responder populations can be identified which can be used to guide clinical strategies and patient stratification.
4. MCTs Provide the Perfect Framework for Biomarker Discovery
As mentioned above, Mouse Clinical Trial data can be leveraged for biomarker discovery, which is ever more crucial for clinical trial success as personalized medicine progresses. MCTs allow sample collection for biomarker discovery, which, following treatment, can be classified into responders or non-responder populations and interrogated for genomic or proteomic differences between the groups.
This allows you to truly capture and leverage patient-to-patient variability preclinically, which is seen universally in the clinical setting.
5. There’s a Different Study Type to Fit a Plethora of Needs
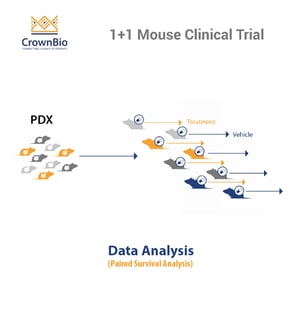
Using an MCT doesn’t limit you to one type study. There are 1+1 and 0+1 designs available (one treatment per one model with and without a comparator arm) dependent on whether you want to more closely mimic a Phase II or Phase I study.
Then there are indication and target driven MCTS:
- indication driven evaluates whether an agent works in only one specific type of cancer, which may be driven by a range of different mutations (like a clinical umbrella trial)
- target drive provides robust target validation, evaluating whether a target/common genetic mutation is present across a range of cancer types, whether the target is engaged, and if there is a downstream effect (like a clinical basket trial)
Overall, this means whatever question you are trying to answer there should be a trial type to suit your needs.
Rethinking the Drug Discovery Paradigm
While it may seem daunting to tear up the rule book and rethink a drug discovery program structure, trialling a method that could drastically decrease attrition rate should always be worth a shot. Choosing to use MCTs should hopefully provide robust, predictive preclinical data to ensure appropriate clinical stratification and improved chance of clinical trial success.
Further reading on Mouse Clinical Trials: