 Tumor-Infiltrating Immune Cells: Explore how the identification and quantification of tumor-infiltrating lymphocytes (TILs) can help evaluating the efficacy of novel immunotherapy agents.
Tumor-Infiltrating Immune Cells: Explore how the identification and quantification of tumor-infiltrating lymphocytes (TILs) can help evaluating the efficacy of novel immunotherapy agents.
Syngeneic Tumor Models in Immuno-Oncology
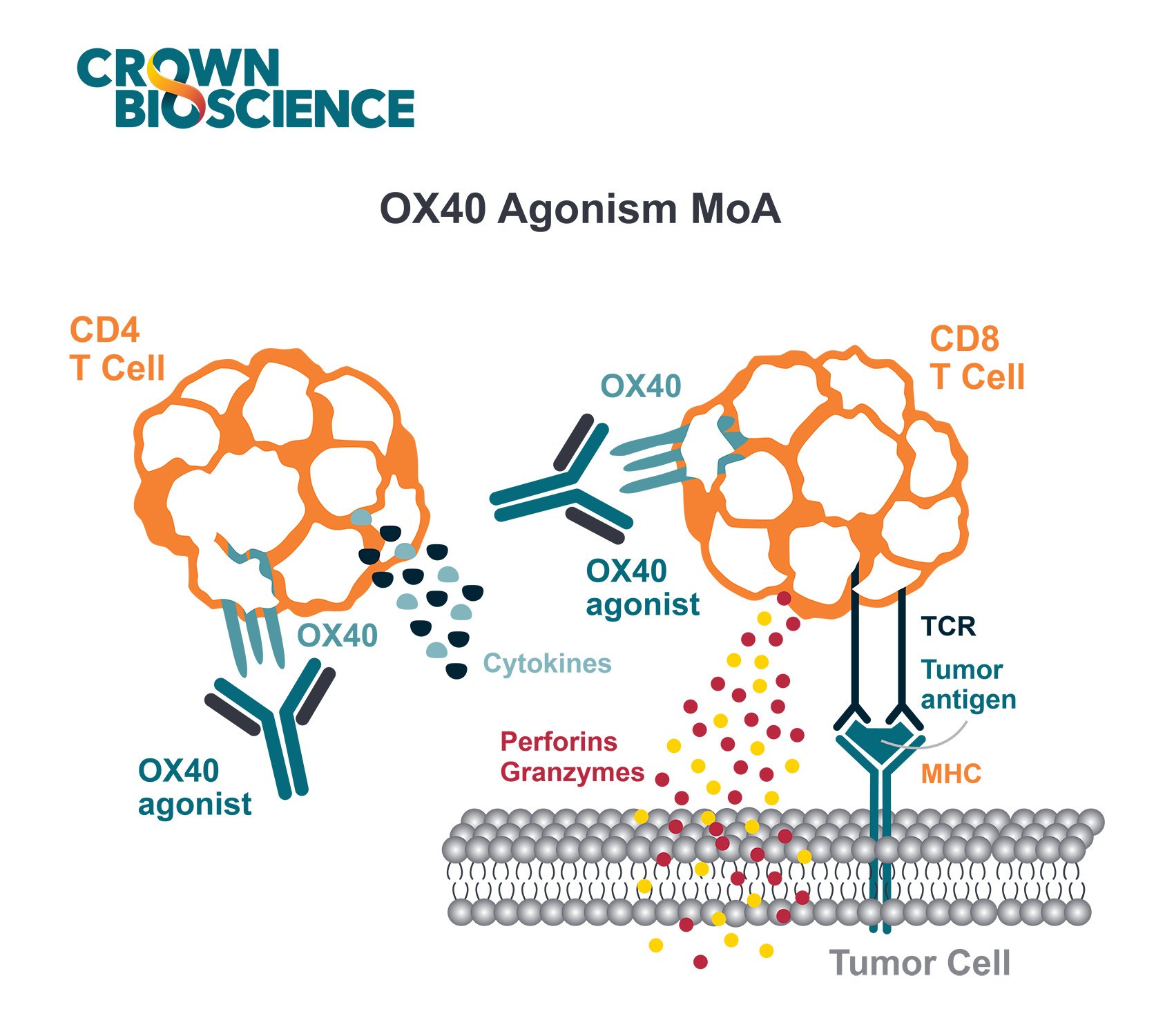
Immuno-oncology (I/O) therapies elicit their activity by modulating the immune system of the host so it can identify and destroy tumor cells. There are several mechanisms through which I/O drugs can modulate the immune system, including:
- Overcoming tumor-induced suppression mechanisms
- Stimulating cytotoxic activity of immune cells
- Increasing the presence of immune cells in the tumor microenvironment
Syngeneic tumor models are widely used in preclinical I/O studies because they are immune-competent and therefore have fully intact and functional immune systems. Studying novel immunotherapy agents in the context of a functional immune system is critical because these models recapitulate the complex interactions that exist between the immune system and tumors. Any alterations of the immune system components, either systemically or at the tumor site, can be monitored in these models. Additionally, studies have demonstrated that these models respond to clinically relevant immunotherapies, both in combination and as monotherapies.
A challenge often encountered with syngeneic tumor models is that they require cross-reactive therapeutics so they can recognize murine targets. However, this challenge can be overcome by using a combination of transgenic syngeneic mice (i.e. mice expressing human targets on their immune cells), syngeneic tumor models overexpressing a human antigen, or using chimeric (human/mouse) antibody-based agents.
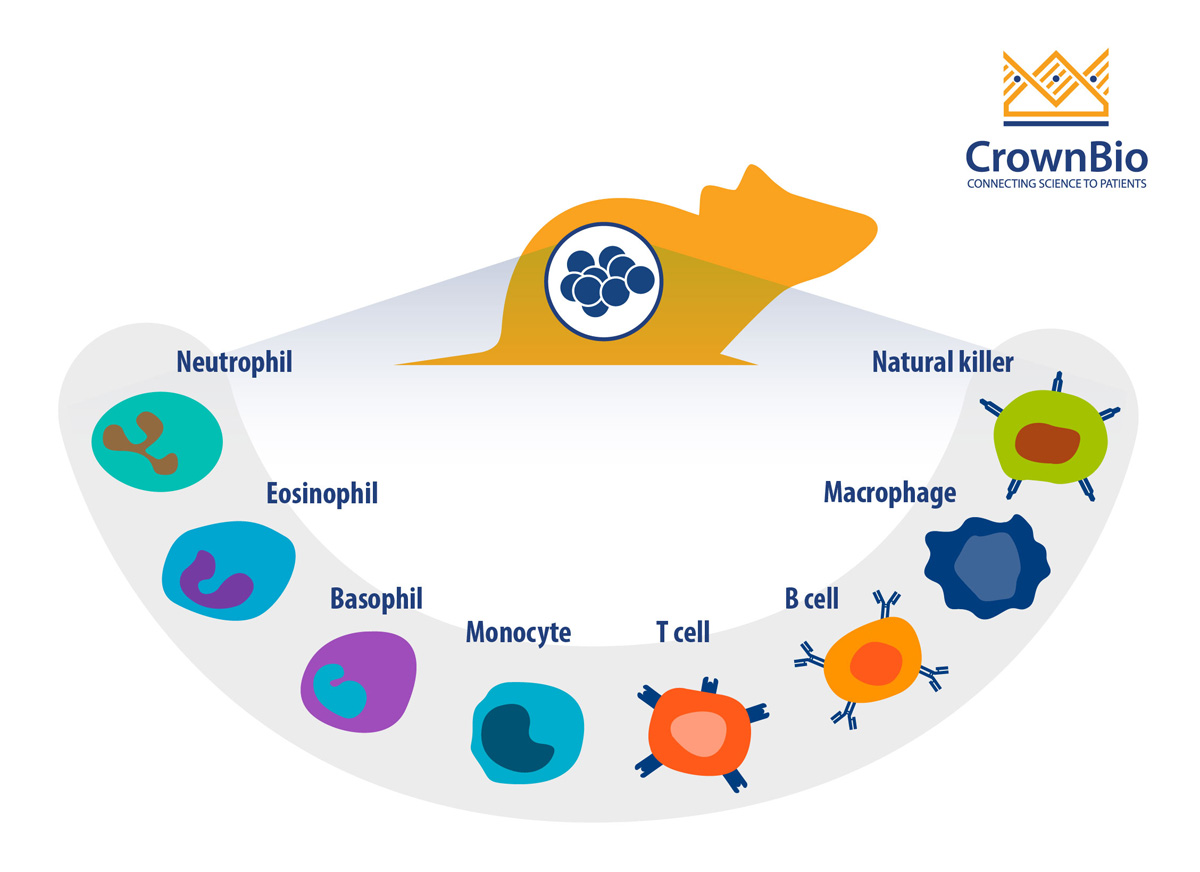
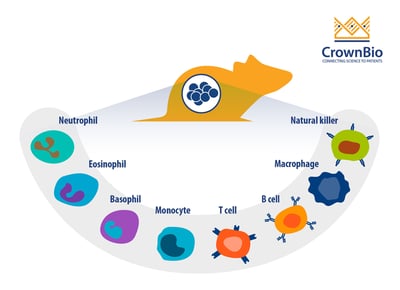
Why Profile the Immune System?
Since I/O therapies elicit their activity by modulating the immune system, it is important to directly profile the immune system to analyze how the I/O agent(s) affect the immune system. This assessment is useful for preclinical efficacy and pharmacodynamic (PD) studies, as immune profiling can determine, or confirm, the mechanism of action (MoA) of an immunotherapy.
Specifically, immune profiling can be used to assess the following parameters/variables:
- Inflammatory status of the tumor model, which can guide tumor model selection for efficacy/PD studies
- Abundance of the therapeutic-relevant effector cells in the tumor model or syngeneic host, which can determine thresholds needed for efficacy/PD studies, as well as selecting appropriate study models
- Increased infiltration of immune cells at the tumor site. This can be determined by profiling the tumor with and without treatment, which can be used to confirm the MoA
- Activation and exhaustion status of immune cells to determine the PD effect of the therapeutic
- Systemic inflammatory responses such as cytokine-release syndrome and cytokine storm, assessed by immune profiling and cytokine profiling of the host
- Off-target/toxic effects, assessed by immune profiling of non-target tissues
How to Perform Immune Profiling
Immune profiling can be achieved using a variety of methods, including:
- Flow cytometry (FC): The most commonly used method to profile tumors and other mouse tissues, including the blood. Advantages of FC include quantifying different types of immune cells and determining the activation state of immune cells, by evaluating the upregulation/downregulation of specific biomarkers, including cell surface proteins and intracellular cytokines. While FC can quantify the immune cells in a tumor/tissue, it does not provide information about the specific location of the infiltrating cells, since the tissues are homogenized for analysis. A further limitation is that the number of parameters that can be analyzed simultaneously is limited based on the equipment available. Newer cytometers with more advanced laser systems may alleviate this problem, but currently we are limited to 12-15 parameters per staining panel.
- Mass cytometry (CyTOF): Similar to FC, antibodies are used to label cellular proteins for analysis in CyTOF. However, instead of having antibodies conjugated to fluorophores, as in FC, CyTOF relies on antibodies that are conjugated to isotopically pure heavy metals (which are normally not present in biological samples). The samples are analyzed by time-of-flight mass spectrometry. The main advantage to CyTOF is the expanded number of parameters than can be analyzed simultaneously due to the low overlap in signal detection among the isotopes, as compared to fluorophores. CyTOF can be used for quantification of immune cell types and activation markers. However, the tissue also has to be processed which results in a loss of spatial information.
- Immunohistochemistry (IHC): This method is a semi-quantitative assay for immune profiling, where tissue sections are directly analyzed, providing spatial information of various immune cells. IHC can provide insights into the MoA/PD effect of the therapeutic, in cases where there are not significant changes in the total number of cells between treated and untreated tissues. IHC is the most cost-efficient method listed here, but it is also the method that measures the lowest number of variables at once.
- Next generation sequencing (NGS): Generally used in combination with one of the immune profiling methods described above, NGS is helpful for developing an in-depth profile of the transcriptome and receptor repertoire of specific immune populations. Genome-wide changes of the immune system – even at the single-cell level – after treatment with immunotherapies can be interrogated using NGS strategies.
- Multiplex Spatial Tissue Analysis (MSTA): Provides a comprehensive and spatially relevant picture of biomarker distribution within a tissue sample. MSTA has come to fruition largely due to advances in digital technologies and new analytical capabilities (e.g. photo-cleavable barcodes and spatially defined UV exposure, and laser/ion-beam ablation of metal-or isotope-labelled antibodies). MSTA can now be used to simultaneously evaluate hundreds of nucleic acid and/or protein markers in a spatially relevant context.
Conclusion
Since syngeneic tumor models have an intact and functional immune system, they are widely used in preclinical studies of novel immunotherapies. To better understand the MoA/PD of novel I/O agents, changes in the immune system components, either systemically or at the tumor-site, are often monitored in these models. Such profiling and analyses of tumor-infiltrating immune cells can provide key insights to enhance the development trajectory of novel I/O agents. The five methods for immune profiling highlighted have their own benefits and limitations which should be taken into consideration before selecting a method for any study. This will maximize the likelihood that the method selected will answer the specific research questions at hand.