 Explore how CAR-T cell therapy is showing promise as a novel treatment strategy for pancreatic cancer.
Explore how CAR-T cell therapy is showing promise as a novel treatment strategy for pancreatic cancer.
The Limited Treatment Options for Pancreatic Cancer
Pancreatic cancer is an indication with limited treatment options. It’s broadly unresponsive to a range of common cancer treatments (e.g. chemotherapy, radiotherapy) as well as targeted agents and immunotherapies such as checkpoint inhibitors. Survival rates have remained stagnant for thirty years, with gemcitabine (approved for pancreatic cancer twenty years ago) remaining the standard of care.
To improve survival rates, new treatment options are needed. CAR-T cell therapy may be part of the answer.
Targeting Pancreatic Cancer with CAR-T Therapy
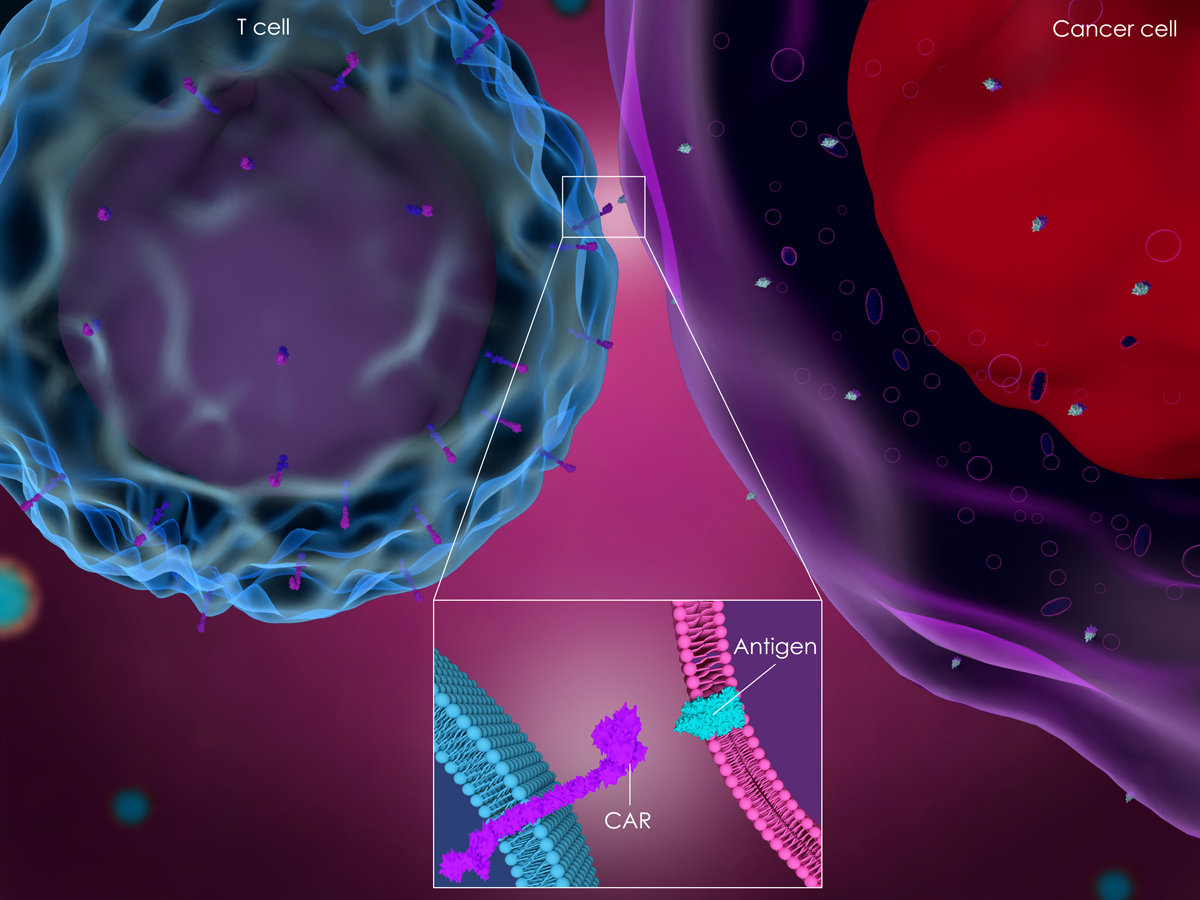
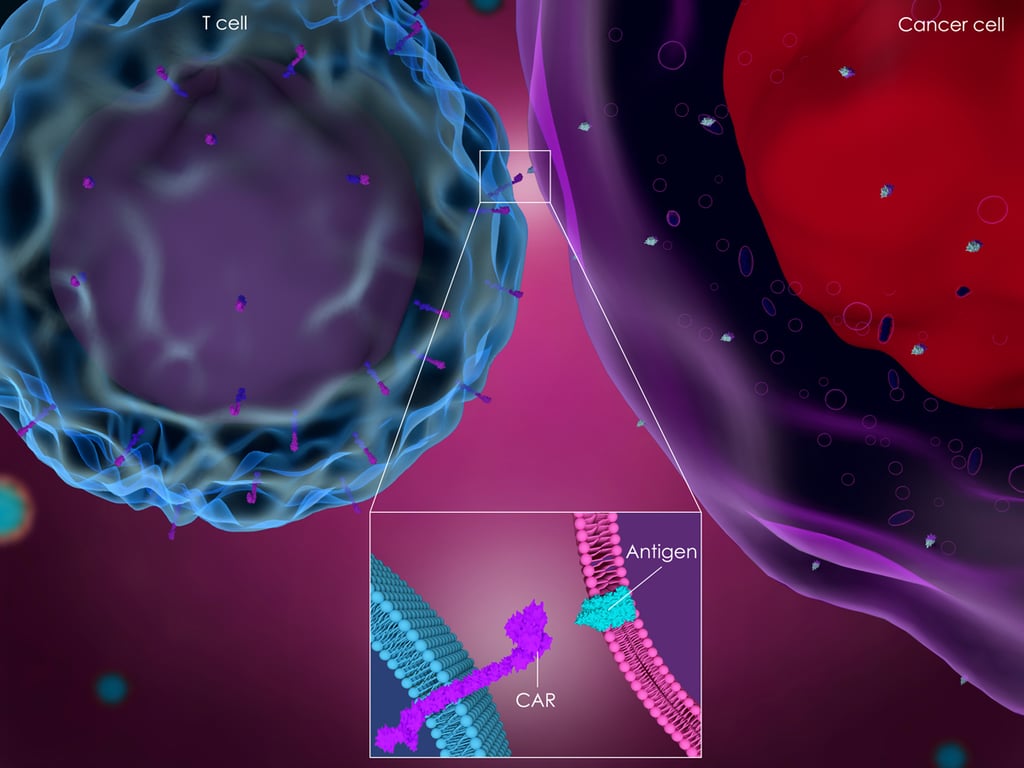
Jiang et al report on the preclinical evaluation of CAR-T cell therapy targeting mesothelin (MSLN) in pancreatic cancer. MSLN is a membrane protein overexpressed in many cancer types, including pancreatic cancers. Expression levels of MSLN are low on normal peritoneal, pleural, and pericardial mesothelial surfaces, therefore providing an attractive oncology target.
MSLN-targeted CAR-T cells have shown antitumor activity in mesothelioma and ovarian cancer models, but this publication is the first detailing pancreatic cancer model treatment.
Generating MSLN Specific-CAR-T Cells for Research
Lentiviral vectors were produced which encoded a variety of CARs, made up of human MSLN-specific scFv and CD28/CD3ζ, CD137/CD3ζ, or CD28/CD137/CD3ζ signaling domains (MSLN-28Z, MSLN-BBZ, and MSLN-28BBZ).
T cells where then transduced by the lentiviral vectors encoding the CAR genes to generate MSLN specific CAR-T cells for testing.
In Vitro Cytotoxicity Studies
First, the CAR-T cells were tested for the ability to recognize and kill MSLN-positive pancreatic cancer cells. These cytotoxicity assays were performed by incubating the transduced CAR-T cells with PANC-1 cells with or without MSLN expression.
In these studies, the MSLN-28Z, MSLN-BBZ, and MSLN-28BBZ T cells all efficiently lysed MSLN-positive pancreatic cancer cells. MSLN-negative PANC-1 cells showed almost no lysis, and mock T cells couldn’t lyse any of the target cells tested, showing the target-dependent activity of the CAR-T cells.
In Vivo Efficacy Testing with Patient-Derived Xenografts
MSLN-28Z and MSLN-28BBZ showed higher tumor lysis ability and greater cytokine secretion than MSLN-BBZ T cells, therefore were carried forward to subsequent in vivo antitumor studies.
These in vivo studies used patient-derived xenograft (PDX) models, which are generated by direct implantation of patient tumors in mice, without an in vitro cell line-establishment stage. The models never adjust to growth on plastic, closely reflecting patient tumors in both histo- and molecular pathology and drug response. PDX therefore provide a more predictive and clinically-representative in vivo model than “conventional” xenografts for efficacy assessment.
MSLN expression on a PDX tumor was confirmed by immunostaining, and the model was treated with MSLN-28Z and MSLN-28BBZ CAR-T cells. The model showed significant suppression of tumor growth and a reduction in mean tumor weight compared to treatment with untransduced T cells. Overall, the results suggest that MSLN-targeting T cells can eliminate pancreatic cancer PDX tumor xenografts in vivo.
This activity, and the tumor suppression, was linked to the in vivo survival of the MSLN-specific CAR-T cells, which trafficked into the mesothelin positive pancreatic cancer PDX tumors.
Summary
This study shows that anti-MSLN CAR-T cells efficiently inhibit the growth of pancreatic cancer in a clinically relevant PDX model. CAR-T cell therapy could have potential as a novel treatment strategy for patients with pancreatic cancer, who currently have limited treatment options and poor disease outcomes.