 Learn how healthy tissue and tumor-derived organoids are used to uncover disease mechanisms, here shining a light on the link between colorectal cancer and vitamin D deficiency.
Learn how healthy tissue and tumor-derived organoids are used to uncover disease mechanisms, here shining a light on the link between colorectal cancer and vitamin D deficiency.
The Problems in Studying Vitamin D Deficiency and Colorectal Cancer Links
Vitamin D deficiency has been linked to an increased risk of colorectal cancer development and mortality, as the intestine is a major target of the vitamin. However, fully understanding these links has, up to now, been somewhat problematic.
The RNA of the vitamin D receptor has been found in higher levels in mouse small and large intestine compared with other tissues. But due to the high renewal of intestinal epithelium, it’s proven impossible to work with these cells long term in vitro, with cell survival limited to a few days. This means an in depth study of vitamin D receptor protein expression and function hasn’t yet been performed in human colon tissue.
A new study has now moved this research forward by comparing healthy colon organoids and patient-derived organoids from colorectal tumors to study vitamin D deficiency effects.
The Benefits of Colorectal Cancer Patient-Derived Organoids
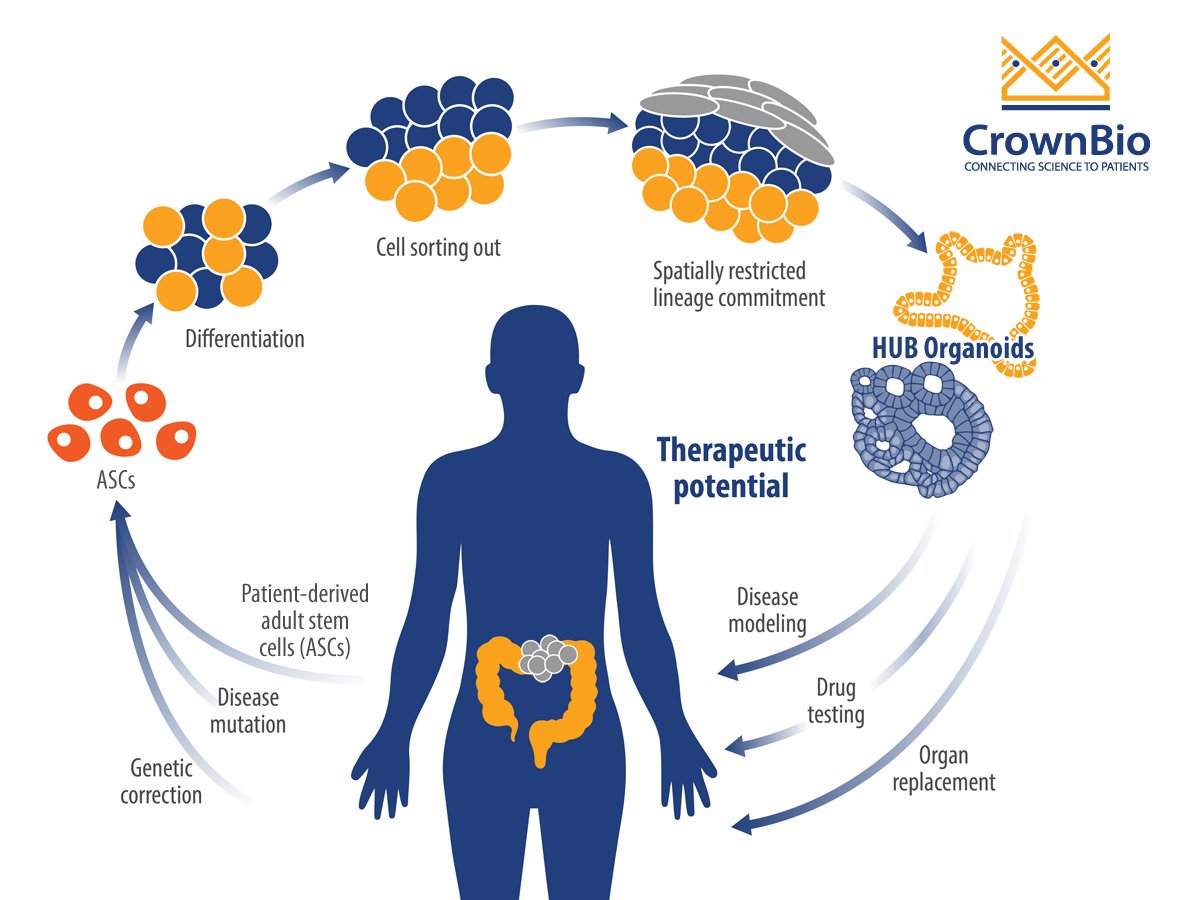
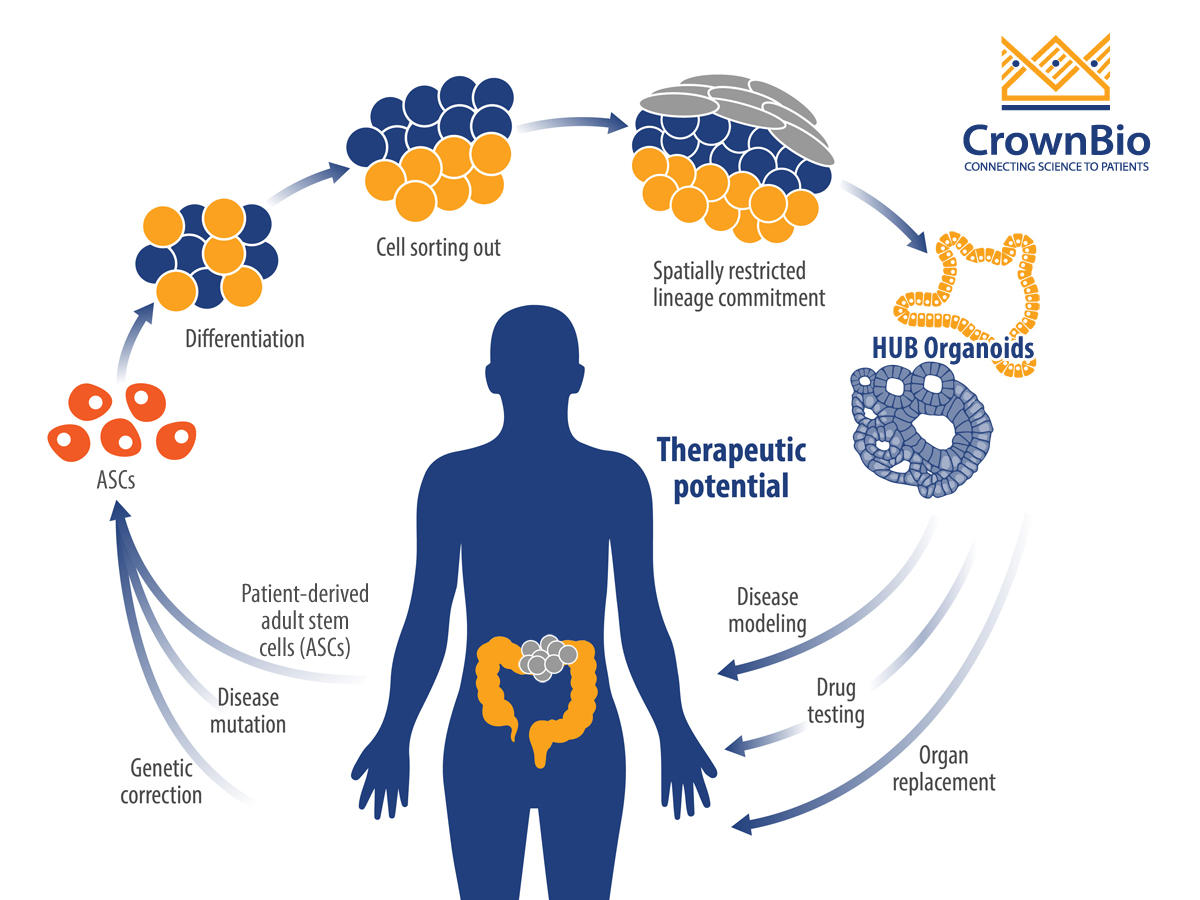
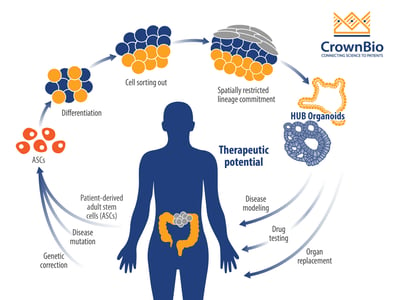
Organoids are a 3D stem cell culture system, that represent miniature imitations of organs including the colon. They’re derived from self-renewing stem cell populations, which differentiate and organize into a miniature form of an organ, keeping genetic stability intact.
Patient-derived tumor organoids show de novo development of 3D self-organizing tissue in vitro from adult stem cells. They can self-renew and expand, and recapitulate somatic copy number and mutation profiles of the parental cancer patients. Organoid genetic and phenotypic stability can be maintained long term, as can organ functionality recapitulating the tissue of origin.
For this study, organoids opened the door to understanding colorectal cancer mechanisms through their unlimited growth potential, providing the time for studies that in vitro cells could not. The research team successfully created a biobank of normal and colon cancer organoids from over 90 colorectal cancer patients, by isolating the stem cell population of the intestine. These organoids were then used to investigate vitamin D effects.
The Different Roles of Vitamin D in Normal and Tumor Organoids
By comparing normal and patient-derived tumor organoids, the researchers studied the effects of vitamin D on organoid transcriptomic profiles and morphology. Vitamin D was found to have contrasting effects on the normal and tumor organoids:
- Normal organoids: Vitamin D downregulates differentiation genes and induces expression of stemness-related genes (e.g. LGR5, SMOC2, LRIG1, MSI1, PTK7, and MEX3A). This supports the maintenance of a stemness phenotype.
- Colorectal cancer tumor organoids: Vitamin D plays a pro-differentiation role.
Overall, the data show for the first time that vitamin D has a regulatory role on human colon stem cells. This suggests a homeostatic effect of vitamin D on colon epithelium, which would have implications for colorectal cancer – with vitamin D potentially playing a protective role.
Challenges with Organoids
While this study shows that organoids can be invaluable for studying disease mechanisms, the researchers have spoken on the challenges of using this new tool. Culture conditions, protocols, and reagents need to be optimized, as does setting up a standard culture for each different organ type. For successful use in translational applications, an expert service provider is often required.
Tumor organoids were first developed by the Clevers’ lab, with these protocols built upon by Hubrecht Organoid Technology (HUB) to provide the gold standard tumor organoids routinely used today. These protocols were followed to generate the tumor organoids used within this study.
Conclusion
Patient-derived tumor organoids and their normal tissue counterparts provide novel 3D research systems enabling new research into colon cancer. Within this study, tumor organoids helped uncover mechanisms behind colorectal cancer, in a way which could not be achieved through regular in vitro studies.