 Review the key questions on HUB tumor organoids from our recent webinar, answered by Dr Sylvia Boj, Hubrecht Organoid Technology (HUB) Scientific Director.
Review the key questions on HUB tumor organoids from our recent webinar, answered by Dr Sylvia Boj, Hubrecht Organoid Technology (HUB) Scientific Director.
Is the Term Organoids used for Models Derived from both Normal and Cancer Cells?
Yes, we use the term ‘organoid’ for the 3D cultures generated using the technology developed in the Clevers lab for either healthy or tumor tissue.
Can Tumor Organoids be Developed from Pancreatic NETs?
Yes, our academic group is developing protocols to expand NETs from pancreas, lung, and intestine.
What's the Success Rate for Liver Cancer Organoid Establishment?
The efficiency of establishing cholangiocarcinomas is relatively high (>70%); however, the efficiency of establishing hepatocellular carcinomas (HCC) is very low. This is because HCC that are resected are quite frequently highly differentiated and have very low proliferative capacity.
Are There Cancer Organoids in Development for Hematological Diseases, e.g. Myelomas?
The HUB Organoid technology developed in the Clevers lab expands epithelial cells. This technology does not support the growth of brain, bone, fat, cartilage, heart, and blood cells.
Have You Tried to Generate Organoids from Circulating Tumor Cells?
No, we haven’t. I think the limitation would be the amount of blood available for isolating circulating tumor cells, and if the amount of tumor cells was enough to establish an organoid culture.
Do you See any Significant Differences Establishing Organoids from Normal vs Tumor Samples?
In tissue like pancreas or breast, where normal organoids are generated from ductal cells, the efficiency of establishing normal organoids is lower due to the low representation of these cells in tissue fragments.
Which Medium Composition are You Using to Culture Organoids from Metastatic Sites? Such as Liver Metastasis from a Primary Tumor in the Colon - is this Cultured in a Medium Composition for Liver or Colon?
For culturing metastatic lesions, we use the medium recipe from the original lesion, so CRC metastatic lesions in the liver are cultured in colon medium.
For Tumor Organoids, Could You be Selecting Clones that Better Adapt to Culture Conditions?
When establishing tumor organoids we use the same protocols and conditions for establishing organoids from healthy tissue. Therefore, if the conditions allow the growth of healthy cells without any selection, we don’t expect any type of selection for tumor cells.
Multiple People have Reported that Normal Organoids often Outgrow and Overtake Cancer Organoids. Have You Experienced this and Do You have any Advice for Enriching for Cancer Organoids?
This is a real problem for prostate cancer organoids. The protocols to grow prostate cells using organoid technology have been established and work very well (Karthaus et al. Cell 2014;(159):163-175; Drost et al. Nat Protoc 2016;(2):347-58). The problem in establishing prostate organoids from cancer models is that resected tissue from prostate tumors is always contaminated with normal/healthy prostate cells and these cells overgrow, so tumor cells are lost over passages.
For establishing other cancer models, ways to prevent the growth of normal cells include removing one growth factor essential for normal cells or adding Nutlin-3, a compound that kills WT TP53 cells. Examples of factors to remove include:
- For colon cancer models Wnt3a is removed from the medium, since the main driver for CRC are APC mutations.
- For establishing pancreatic cancer models EGF can be removed from the medium, as the main driver for PDAC are KRAS mutations.
- For establishing high grade ovarian cancer models Nutlin-3a can be added to the medium, as the main driver for HG ovarian tumors are P53 mutations.
For other models such as breast or lung cancers, where there is not a main driver for tumor formation, if there is enough material then a matrix medium in 48 well plates is an option. This can remove EGF (for KRAS or EGFR mutants), add Nutlin3a (TP53), remove Noggin or TGFBi (BMP or TGFB pathway), etc.
Another key aspect is pathologist involvement, to provide as pure a tumor sample as possible for organoid establishment.
Have you Checked to what Extent the Genomic Alterations from Original Patients are Maintained in the Organoids over Time? Are they Preserved?
In the first publications on cancer biobanks from our academic group (i.e. van de Wetering et al. Cell 2015;(161):933-945, Sachs et al. Cell 2018 (172):373-386), the direct comparison of mutations from the original tumors and derived organoids shows an overlap higher than 80%.
In the case of identifying mutations in either only the biopsy or organoid, this is explained by the fact that the tumor piece used for either sequencing or generating organoids is not the same. Also, tumor organoids represent the epithelial compartment of the tumor (tumor cells) and so low frequency mutations can be identified during sequencing. Within the tumor sample though, other cell types are also sequenced so these mutations can be missed.
Do you Genetically Pre-Screen Organoids? I.e. if We Wanted a Cancer Model Involving a Specific Cancer Mutation can we obtain those Organoids Specifically
HUB cancer organoid biobanks are being characterized at the DNA and RNA level, so genetic landscape and gene expression are known for each organoid model.
Can you Clarify why at Least 1 of 4 Clones from the Same Patient Treated with a Specific Compound Shows Resistance?
Each clone was generated from a different region of a primary CRC tumor. When each clone was exposed to different compounds, it was observed that at least one clone was resistant to the drug. This suggests that drug resistance is intrinsic to the tumor and not necessarily a response to treatment. To know more, the data presented is part of publication from our academic partner (Roerink et al. Nature 2018;(556):457-462).
Do you Check Patient-Derived Cells and Related Organoids for Multi-Drug Resistance?
Yes, we are evaluating whether resistant clones can be identified in organoids generated from untreated tumors. We are also collecting samples from recurrent lesions from primary lesions which organoids were generated from, to understand the mechanism of resistance.
Do you Have any Examples of Successful Replication of Human Clinical Trial Observed Efficacy by Testing Drugs In Vitro with Patient-Derived Organoids?
HUB is currently evaluating the predictive value of organoids for metastatic CRC patients. Last year, results from The Institure of Cancer Research comparing organoids (generated with our protocols) and clinical response in CRC patients was published in Science. The results were quite amazing, observing a 100% negative predictive value and 85% positive predictive value.
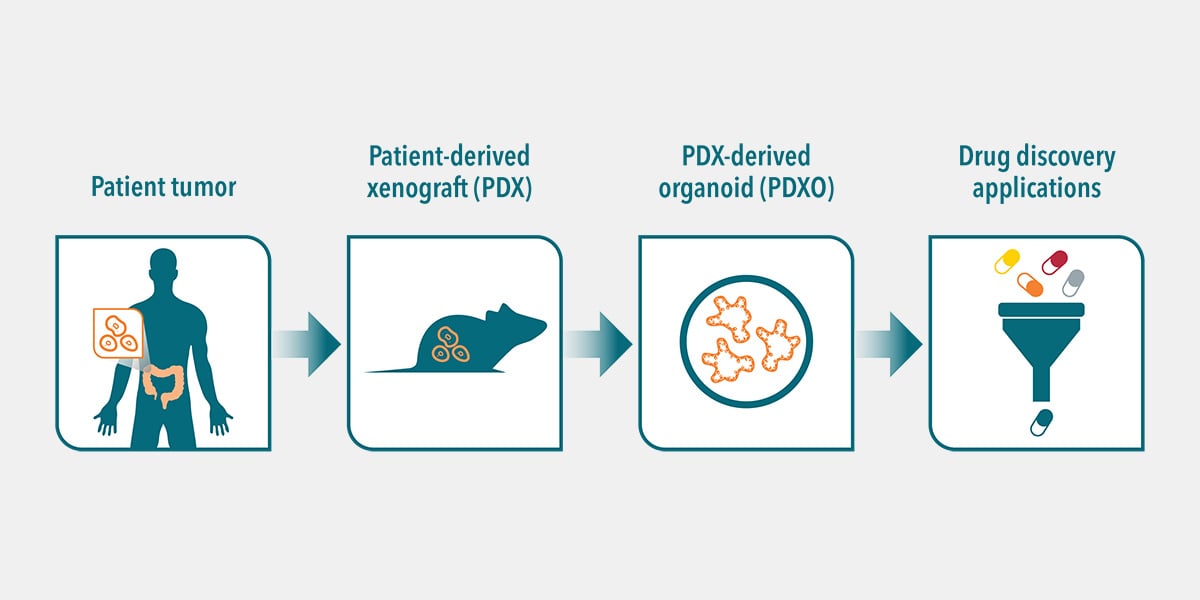
What is the Minimum Number of Organoids used to Establish Xenograft Tumors in Mice?
The number of organoids needed to establish a xenotransplant is comparable to what is needed for cell lines. In one of our collaborations, 500,000 cells (~32.000 organoids) were enough to generate a PDX model.