 Learn more about the need for large-scale cancer immunotherapy screens and the different immunocompetent models available for screening.
Learn more about the need for large-scale cancer immunotherapy screens and the different immunocompetent models available for screening.
Why Are Large-Scale Screens Needed for Immunotherapy Development?
Immunotherapies and combination regimens are complex in nature. Their comprehensive preclinical testing in relevant immuno-oncology models is critical to evaluate efficacy and pharmacodynamics (PD), as well as uncover any potential safety issues or optimize treatment regimens before clinical trials.
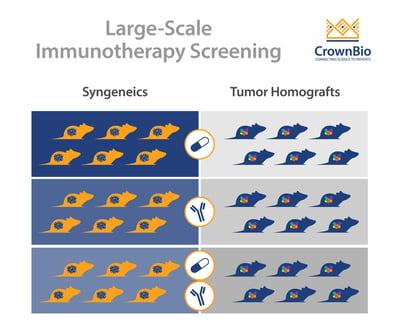
One route for evaluating new immunotherapies and combination regimens is through large-scale I/O screens. These screening platforms are increasingly relevant for a number of reasons, including variability due to individual immune signatures and a need for a variety of model types.
Within preclinical studies, inter- and intra-group variability occurs due to the complexity of the immune system and its interactions with the environment and tumor in a living organism. For example, although mice used within a syngeneic study have the same genetic makeup, individual mice have their own unique immune signatures. This inherent variability results in varying responses to the same treatment, calling for scalable preclinical models that can provide sufficient ‘n’ for meaningful analyses.
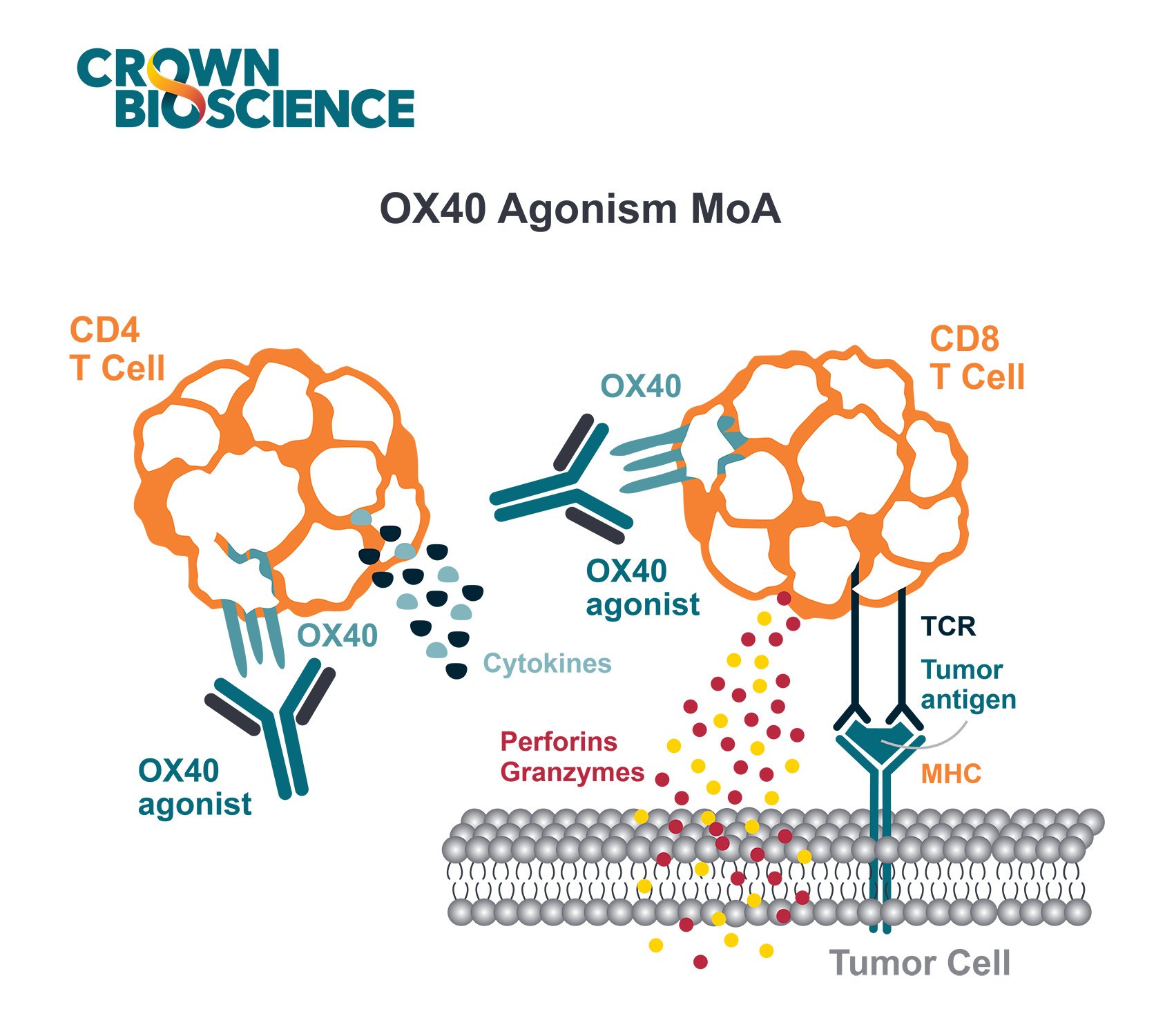
There’s also a need for a variety of model types to understand the effectiveness of your I/O agent in diverse molecular and tumor immune microenvironments. We don't always know why some patients respond and others don't. Therefore, using a variety of model types with different molecular drivers and tumor immune environments in large-scale allows the investigation of mechanism of actions, PD effects, and potentially discovery of predictive biomarkers.
Immunocompetent Models for Immuno-Oncology Screening
Syngeneic Models
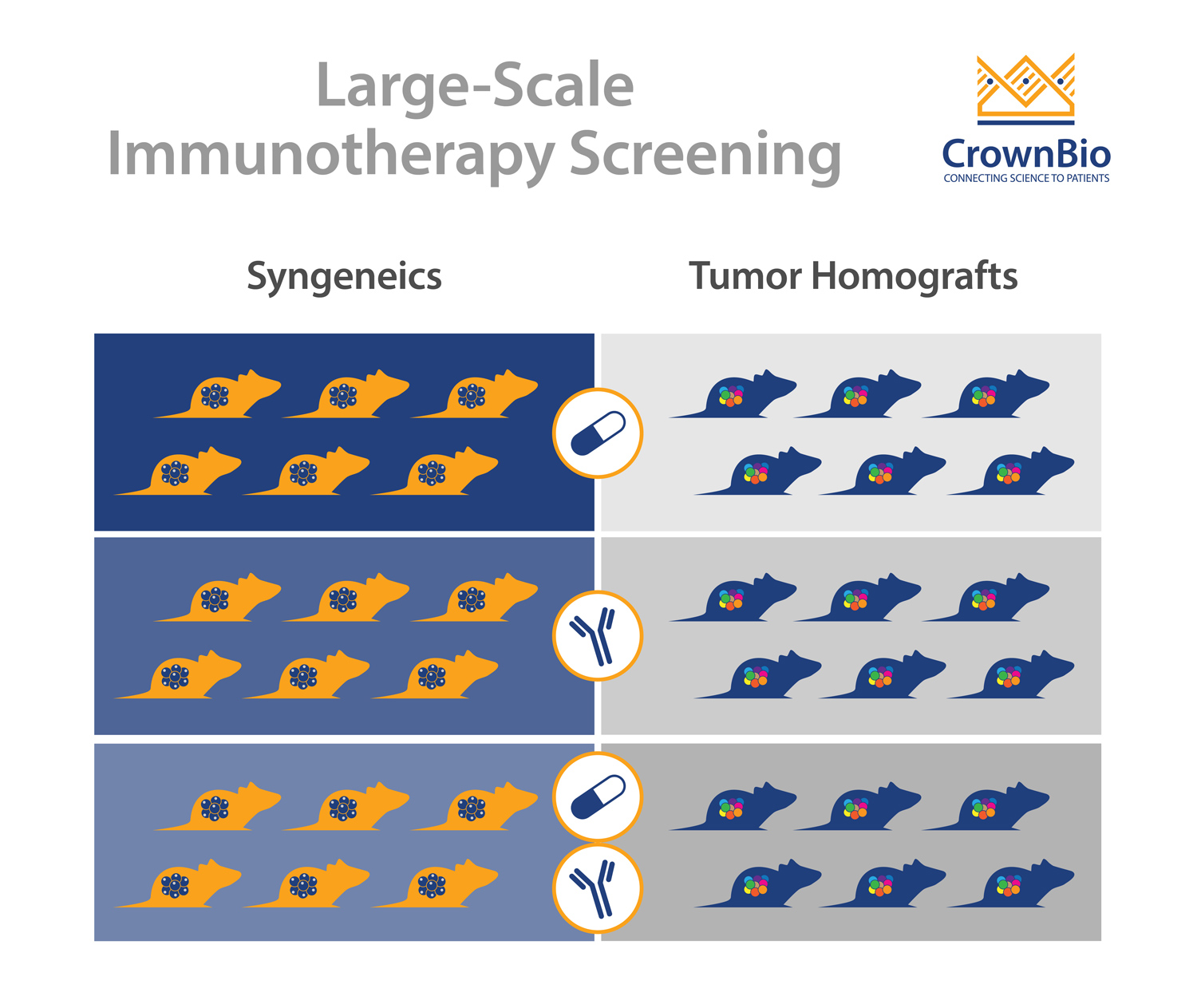
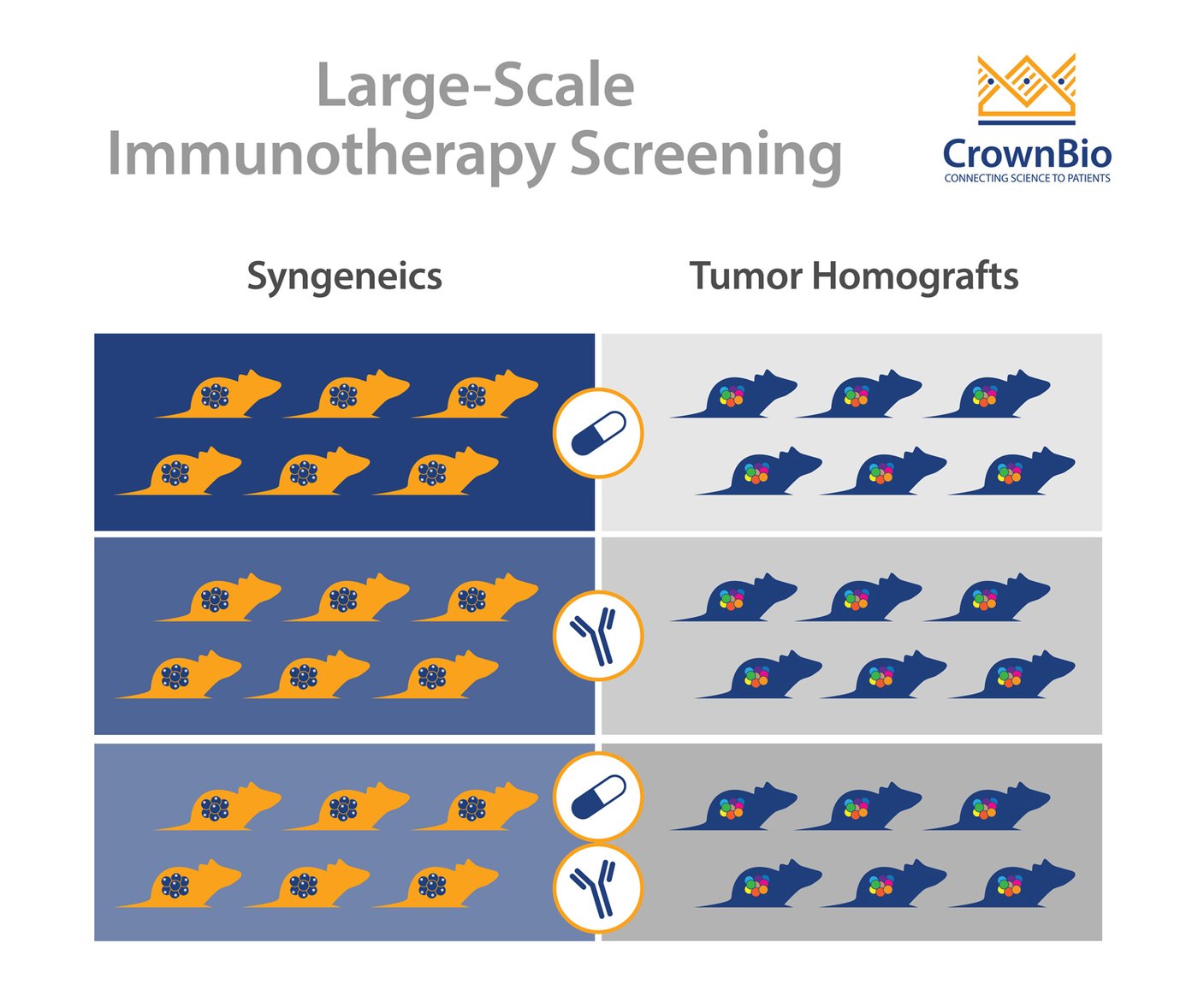
The main model type being used for immunotherapy screens are syngeneic models, still often considered the “workhorse” of I/O studies. Syngeneics are mouse cancer cell lines grown in a fully immune competent host with the same genetic background.
One key advantage of syngeneic models is that they can be quickly established in large numbers. In addition, there is a wealth of historical data available for comparison, as syngeneic models have been around for several decades.
Most importantly, syngeneic models represent the range of immune profiles observed in the clinic, making them an ideal platform for large-scale screening of immune modulating agents.
Syngeneic models have been widely used to perform proof of concept studies by testing the in vivo efficacy of a compound in order to identify responder and non-responder models. They can also be used to assess the PD effect of a compound on the immune profile of tumor, blood, and immune relevant organs, uncover predictive biomarkers, and understand mechanisms of action.
The Need for Alternative Immunocompetent Models
While syngeneics do provide a good start pointing for I/O screening, the hard truth is that there are only a limited number of models available. As they’ve been passaged in vitro during derivation from immortalized cell lines there’s also the risk of genetic drift and enhanced tumor mutational burden.
For some indications, syngeneics are simply not the best models for efficacy testing due to a lack of specific, disease relevant mutations and TME. For instance, Pan02 is a commonly used pancreatic cancer syngeneic model, but it lacks KRAS mutations which are seen in >90% of human pancreatic cancers.
Tumor Homograft Models
Tumor homograft models provide a novel new alternative for immunotherapy screening. They are derived from GEMM or carcinogen-induced tumors that harbor clinically-relevant oncogenic driver mutations such as KRAS, p53, and PTEN. Similar to traditional syngeneic models, tumor homografts are grown in immunocompetent hosts of the same genetic background, allowing testing of surrogate and cross-reactive immunotherapy agents.
Unlike syngeneic models, tumor homografts are never passaged in vitro. Instead they are passaged solely in vivo, preserving tumor architecture relevant to the original TME. And unlike traditional GEMM models, tumor growth can be synchronized across several animals to evaluate tumor response to treatment, making them amenable to large-scale studies.
Due to the patient-relevant mutations and TME provided by these models, tumor homografts help overcome the challenges faced by traditional syngeneic models, serving as the ideal complement in a large-scale screening program.
Summary
Large-scale immunotherapy screens provide a fast and efficient method to evaluate efficacy, PD effects, and to identify responder models or markers. By harnessing the variety of immunocompetent preclinical models available for these screens, you can be better prepared and progress your I/O program with more confidence.