Tumor Organoid Q&A: PDXO Models and Immuno-Oncology Applications
 Review the key questions and answers on PDX-derived organoids (PDXO) and tumor organoid immuno-oncology applications from our recent webinar on 3D in vitro drug discovery.
Review the key questions and answers on PDX-derived organoids (PDXO) and tumor organoid immuno-oncology applications from our recent webinar on 3D in vitro drug discovery.
PDXO Models and Characterization
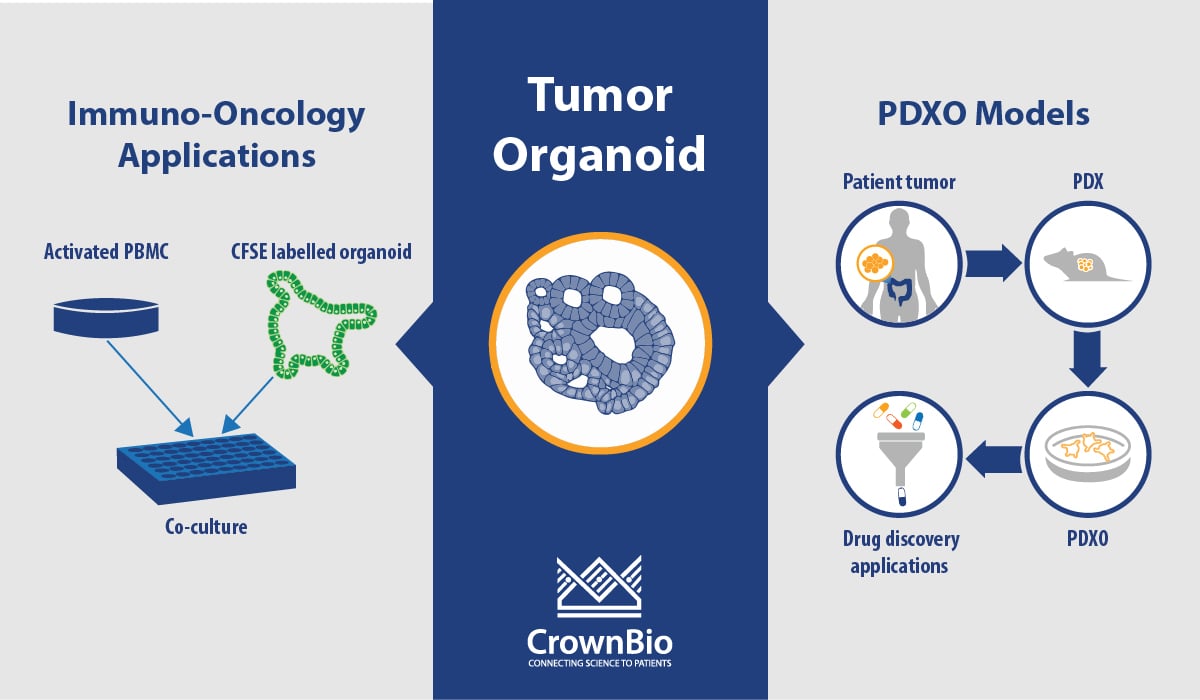
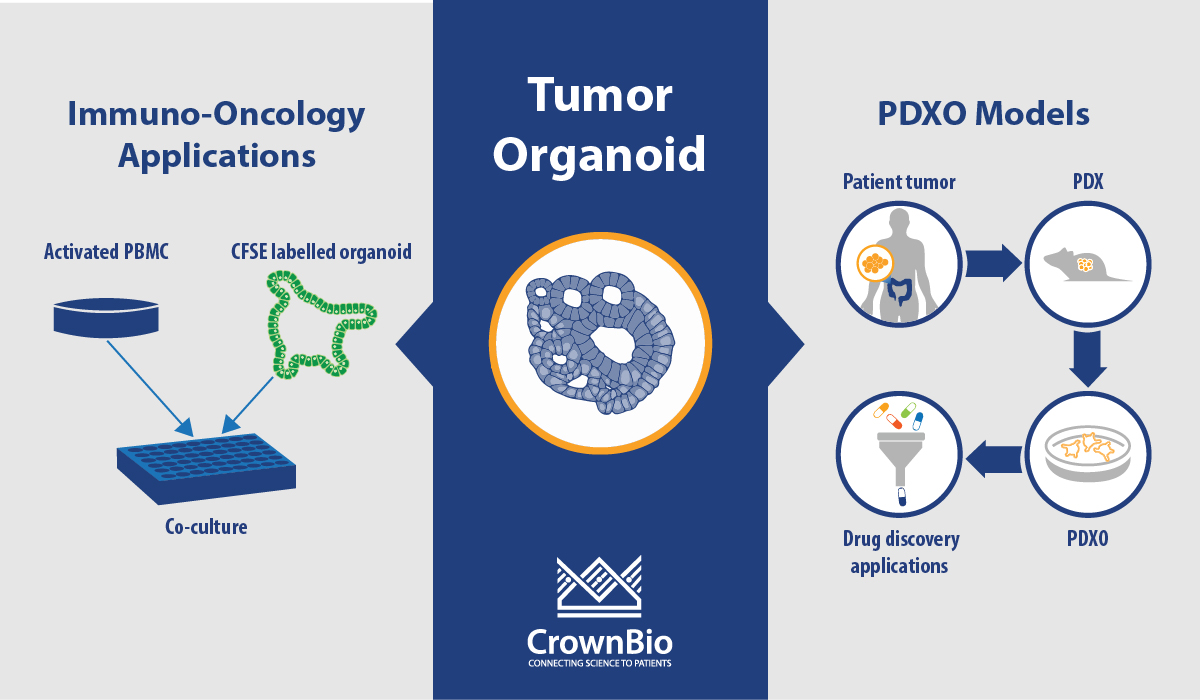
What's the Difference between PDX-Derived Organoids (PDXO) and 3D Cell Culture from PDX Models?
PDXO are organoids that are grown using the same protocols, and develop following the same biological principles, as organoids derived directly from patient tissue. In contrast, 3D culture from PDX models uses PDX material dissociated and admixed with ECM to perform an assay which cannot be propagated any further.
How Frequently is Organoid Characterization Performed? If an Organoid is Passaged Through 10+ Passages (for example)?
We work to build a master cell bank first, and this will vary in terms of passage for each model. The master cell bank organoids are then characterized once we know that the growth of an organoid can be achieved and has been SNP verified. From this, a working cell bank is developed and again SNP verified.
What is the Correlation between PDXO and Parental PDX Model Response? Are Some Indications Better than Others? Do Chemotherapeutics Correlate Better or Worse than Targeted Therapies, or Biologics vs Small Molecules?
We have correlated PDXO IC50 with the TGI for the parental PDX model. In depth data was shown in a previous webinar available from our website.
The correlation is based on 59 matched sets (this number will increase as more data is generated) across 4 cancer types, 11 different models, and 12 different test agents. We’re looking to dissect this out further into cancer types, therapeutic class, and mutational status as we generate more data, and hope to have more clarity on which is more predictive.
Are you Going to Offer Something Analogous to Your OmniScreen™ Cell Based Screening Service, Where Costs are Reduced by Multiple Participants Screening against Selected Models?
At the moment we are focussing on our three main screening options:
- Library screening to identify lead compounds and improve the patient-relevance of hit finding
- Efficacy quantification to compare agent efficacy across patients and compounds and predict in vivo response
- Combination strategy screening to evaluate (multiple) combination regimens and find optimal in vivo dosing regimens
and we have made these studies as cost effective as possible. We can also adapt screens to have multiple readouts.
Are the Only Assay Readouts you provide CTG? Can You, for example, do Microscopy?
For the purpose of the screening options we have streamlined this offering for CTG but yes we can provide other readouts.
Organoids and Immuno-Oncology Applications
As Tumor Mutational Burden (TMB)/Neoantigen Production is Key for Predicting Positive Efficacy for some Solid Tumors, how is TMB Evolved in Organoid Models?
We have WES on our PDX and PDXO models, however we don’t have matched normal tissue to assess TMB. We can assess the mutational load relatively within the cancer type, as well as microsatellite instability or DNA mismatch repair.
As organoids have been shown to retain mutational concordance from the corresponding tumor in vivo, then we are confident that the mutational landscape representation is sustained in the organoids.
Have you Ever Checked the Immune Population within Tumor Organoids?
The HUB Organoid method develops the epithelial compartment, so there are no immune cells present.
Can you Assess Immunotherapies In Vitro with PDXO or PDO?
We are currently developing a co-culture assay and conditions, and we shared some of our allogenic T cell co-culture approach in a poster at the AACR-NCI-EORTC meeting last year (available here). In these studies, we used healthy PBMCs pre-activated with anti-CD3 and anti-CD28 for 3 days before co-culture with organoids. Validation of this assay with immunotherapies is still ongoing.
We also provide tumor organoid killing assays by NK-mediated ADCC killing again using PBMC from healthy donors, and we’re also providing organoids for testing CAR-T cell therapies.
In your Assay, Do you Only See the Endpoint Result of T Cell Co-Culture with Cancer Organoids? Or Do you also Evaluate the T Cell Migration Dynamics or Accumulation Dynamics on Spheroids?
The assays are designed to assess organoid killing, and also assess the tumor reactivity of the T cells, so these are very much co-cultures with admixing at different ratios, without studying migration.
Contact us with any further questions on our organoid platform and models.
Cite this Article
Kumari, R., (2020) Tumor Organoid Q&A: PDXO Models and Immuno-Oncology Applications - Crown Bioscience. https://blog.crownbio.com/tumor-organoid-qanda-pdxo-immuno-oncology-applications